Abstract
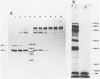
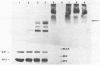
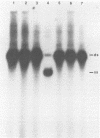
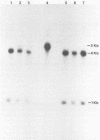
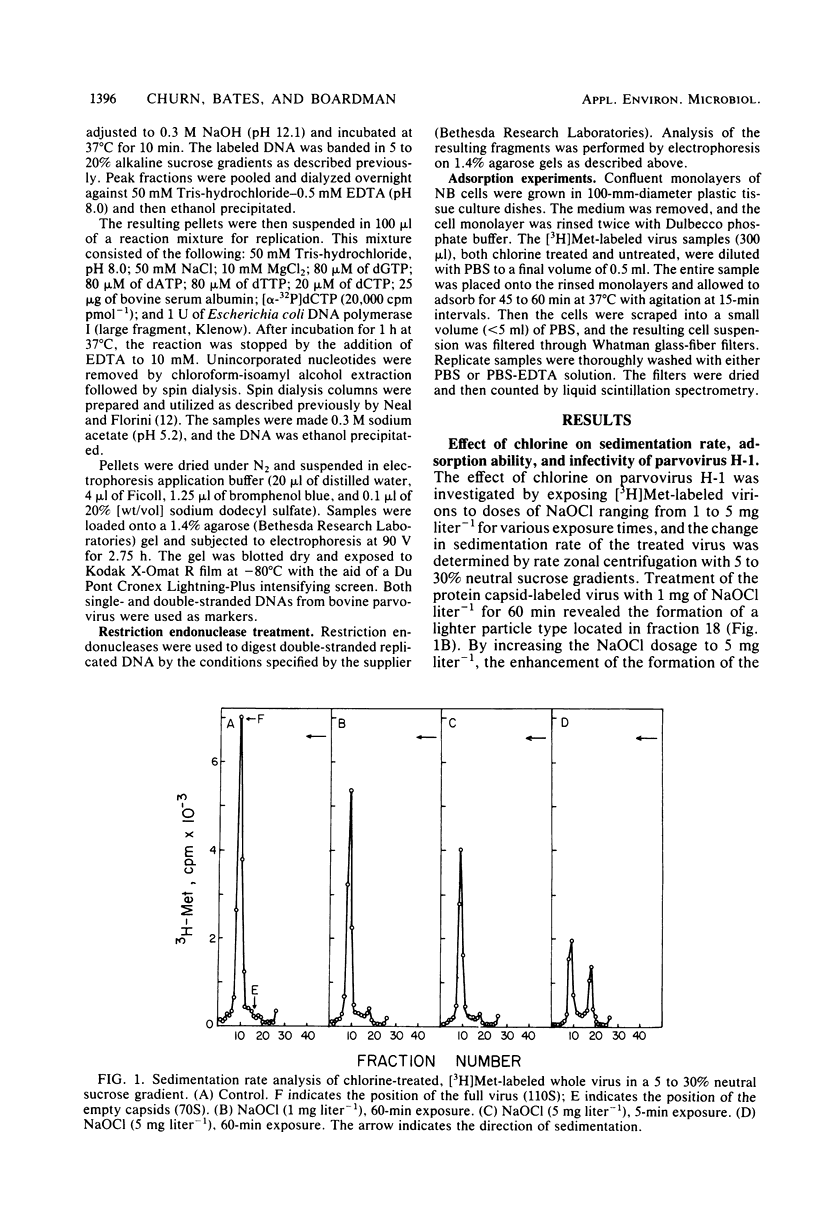
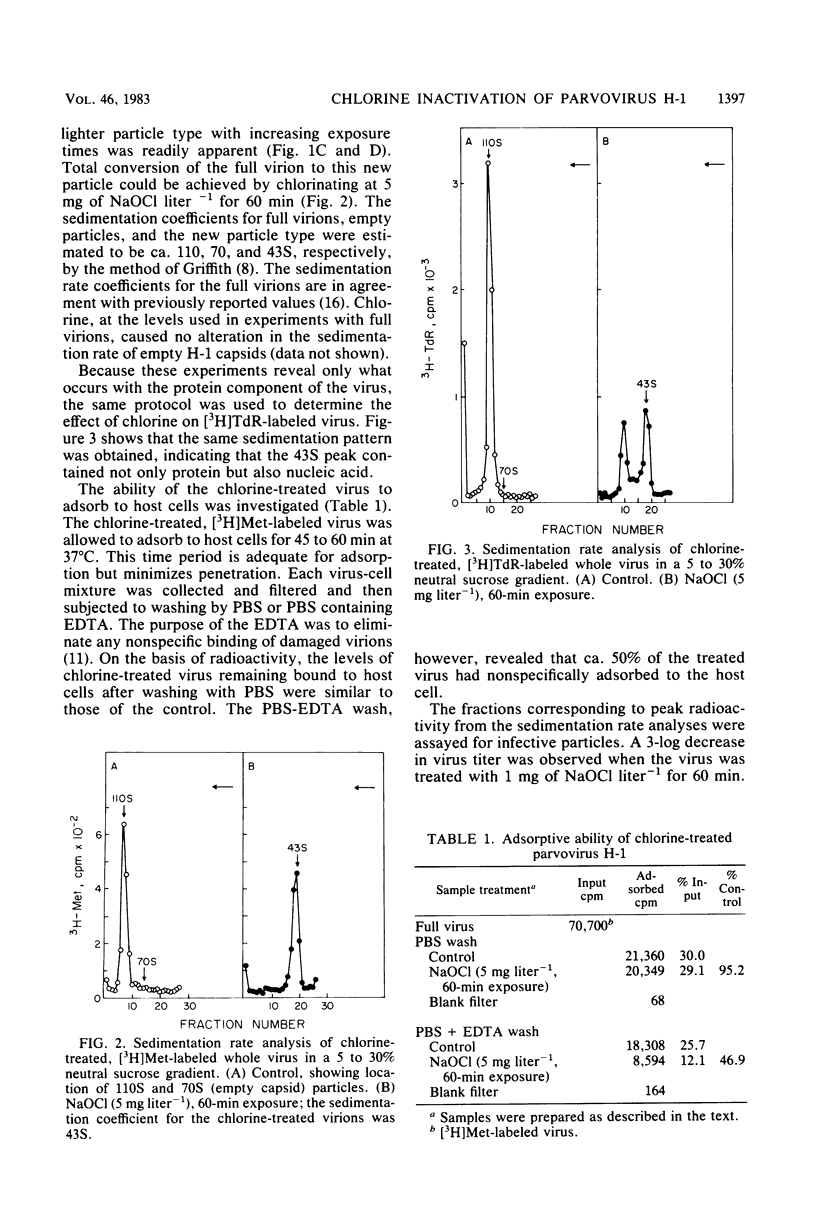
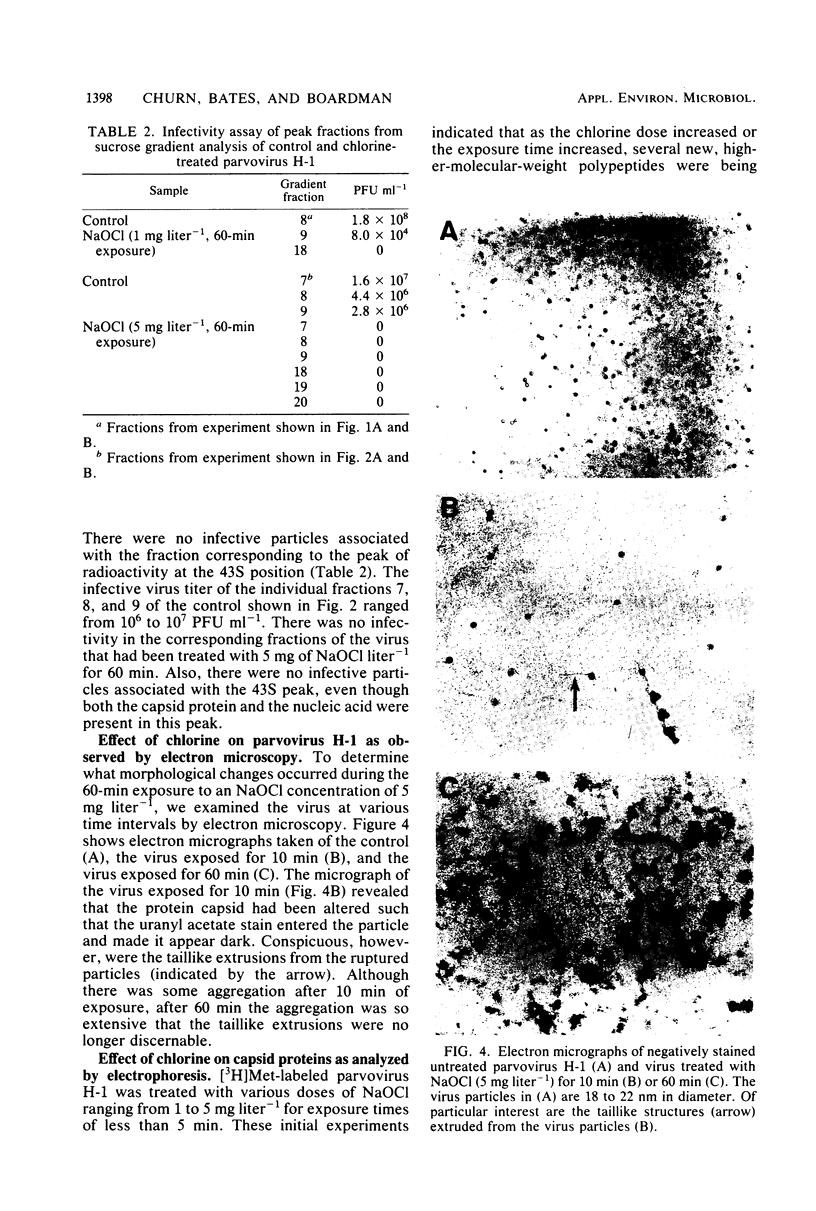
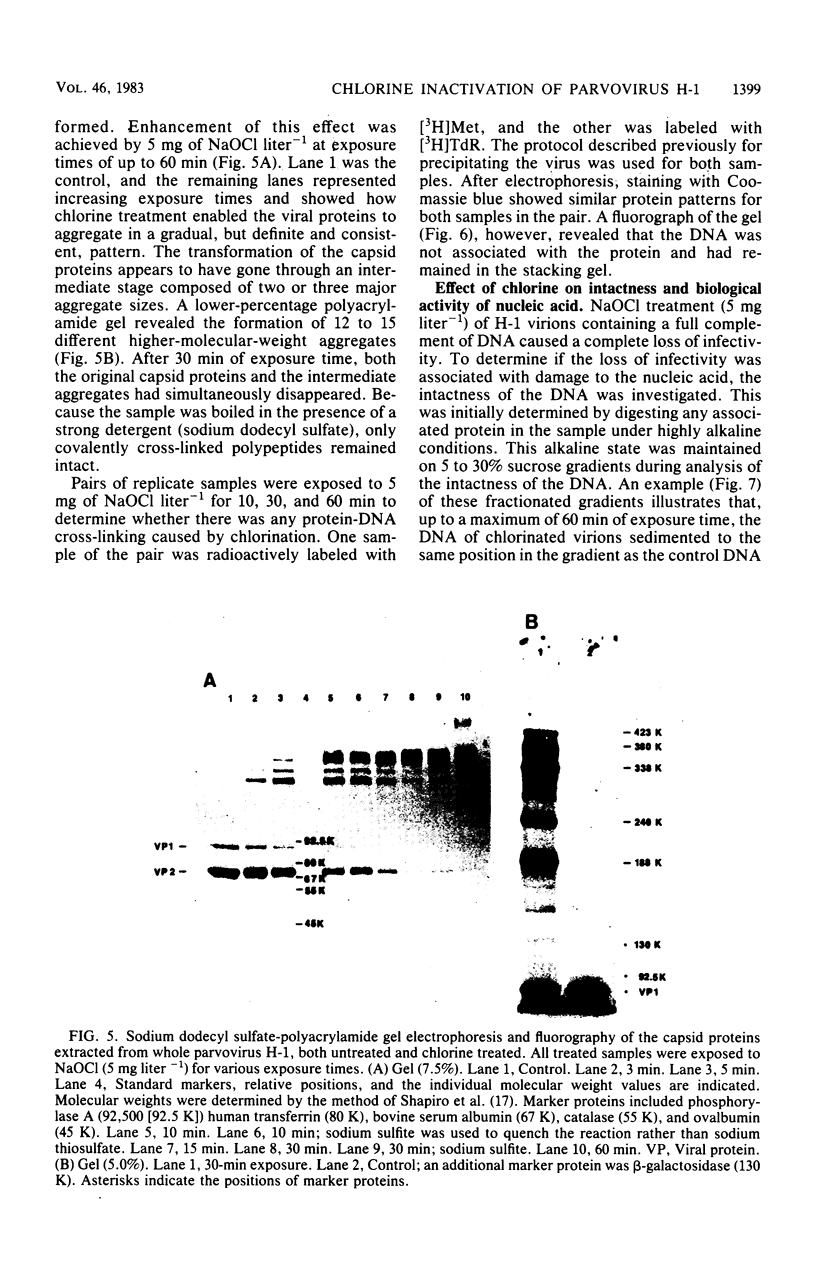
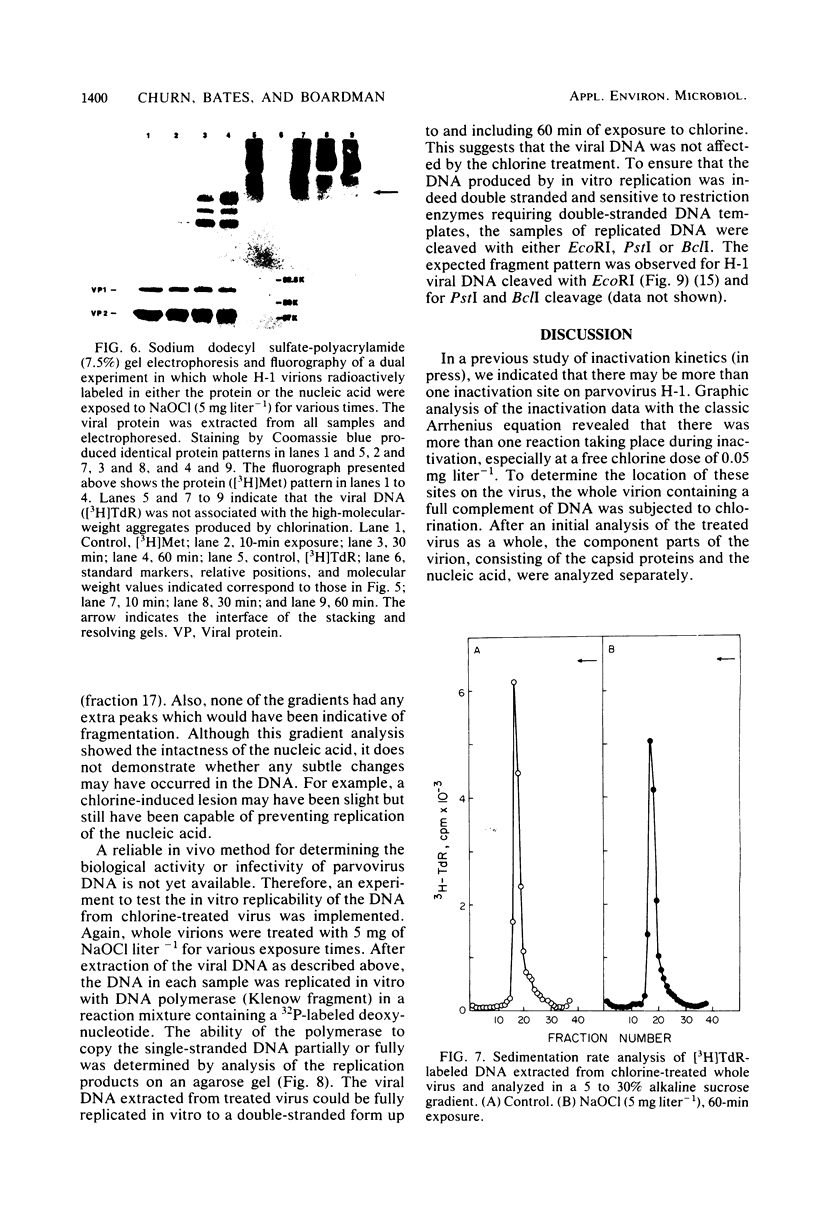
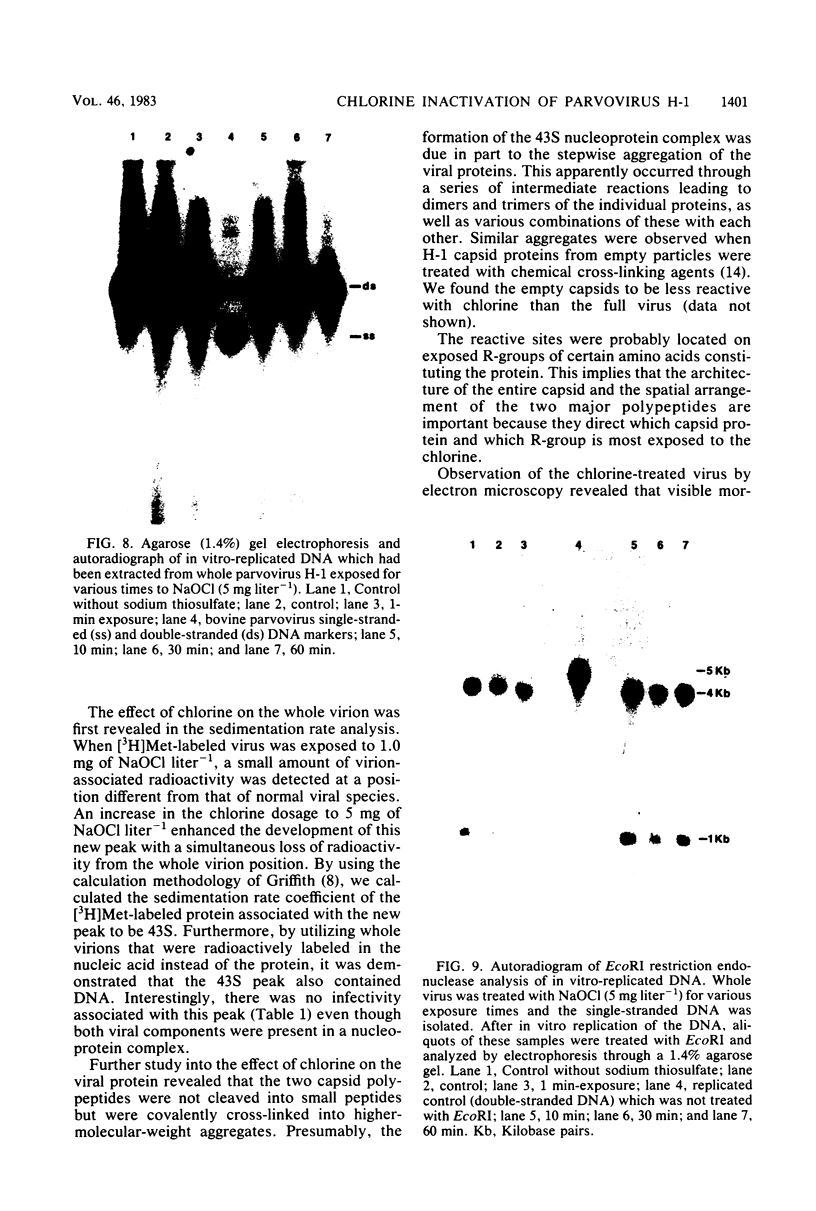
An investigation was undertaken to determine the effect of chlorine on a small DNA-containing enteric virus. Parvovirus H-1 was exposed to sodium hypochlorite in a phosphate-buffered saline solution at pH 7. Then, the whole virion, the protein capsid, or the nucleic acid was subjected to analysis. The sedimentation rate of the chlorine-treated whole virus decreased from 110S to 43S. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the virus demonstrated the formation of higher-molecular-weight aggregates resulting from covalent cross-linking of the capsid proteins. Electron microscopic examination revealed that the DNA was extruded as a taillike structure which remained attached to the virus particle. Furthermore, the DNA was intact and still capable of in vitro replication. The adsorption of the chlorine-treated virions to host cells was inhibited, presumably due to the effect of chlorine on the particular spatial arrangement of the capsid proteins required for adsorption. Specific sites on these proteins had become highly reactive, indicating that the initial action of chlorine on parvovirus H-1 was on the viral capsid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez M. E., O'Brien R. T. Effects of chlorine concentration on the structure of poliovirus. Appl Environ Microbiol. 1982 Jan;43(1):237–239. doi: 10.1128/aem.43.1.237-239.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Engelbrecht R. S., Weber M. J., Salter B. L., Schmidt C. A. Comparative inactivation of viruses by chlorine. Appl Environ Microbiol. 1980 Aug;40(2):249–256. doi: 10.1128/aem.40.2.249-256.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda N. M., Trieff N. M., Stanton G. J. Inactivation of poliovirus by chloramine-T. Appl Environ Microbiol. 1981 Sep;42(3):469–476. doi: 10.1128/aem.42.3.469-476.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledinko N. Plaque assay of the effects of cytosine arabinoside and 5-iodo-2'-deoxyuridine on the synthesis of H-I virus particles. Nature. 1967 Jun 24;214(5095):1346–1347. doi: 10.1038/2141346a0. [DOI] [PubMed] [Google Scholar]
- Linser P., Bruning H., Armentrout R. W. Specific binding sites for a parvovirus, minute virus of mice, on cultured mouse cells. J Virol. 1977 Oct;24(1):211–221. doi: 10.1128/jvi.24.1.211-221.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- O'Brien R. T., Newman J. Structural and compositional changes associated with chlorine inactivation of polioviruses. Appl Environ Microbiol. 1979 Dec;38(6):1034–1039. doi: 10.1128/aem.38.6.1034-1039.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso P. R. Analysis of the protein-protein interactions in the parvovirus H-1 capsid. J Virol. 1983 Apr;46(1):94–102. doi: 10.1128/jvi.46.1.94-102.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass H. L., Salzman L. F., Klorman R., Kaskey G. B., Klein R. H. The effect of distraction on acute schizophrenics' visual tracking. Biol Psychiatry. 1978 Oct;13(5):587–593. [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. IX. Physical mapping studies of the H-1 genome. J Virol. 1977 May;22(2):446–458. doi: 10.1128/jvi.22.2.446-458.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Toolan H. W. The picodna viruses. H, RV, and AAV. Int Rev Exp Pathol. 1968;6:135–180. [PubMed] [Google Scholar]