Abstract
A rapid and reproducible method of inhibiting the expression of specific genes in mosquitoes should further our understanding of gene function and may lead to the identification of mosquito genes that determine vector competence or are involved in pathogen transmission. We hypothesized that the virus expression system based on the mosquito-borne Alphavirus, Sindbis (Togaviridae), may efficiently transcribe effector RNAs that inhibit expression of a targeted mosquito gene. To test this hypothesis, germ-line-transformed Aedes aegypti that express luciferase (LUC) from the mosquito Apyrase promoter were intrathoracically inoculated with a double subgenomic Sindbis (dsSIN) virus TE/3′2J/anti-luc (Anti-luc) that transcribes RNA complementary to the 5′ end of the LUC mRNA. LUC activity was monitored in mosquitoes infected with either Anti-luc or control dsSIN viruses expressing unrelated antisense RNAs. Mosquitoes infected with Anti-luc virus exhibited 90% reduction in LUC compared with uninfected and control dsSIN-infected mosquitoes at 5 and 9 days postinoculation. We demonstrate that a gene expressed from the mosquito genome can be inhibited by using an antisense strategy. The dsSIN antisense RNA expression system is an important tool for studying gene function in vivo.
The incidence of mosquito-borne diseases is increasing among animal and human populations worldwide (1). Reasons for this increase are multifactorial and include the demise of mosquito control programs and increased insecticide resistance (2, 3). New tools and approaches to characterize gene function in vectors are critical for developing innovative control strategies for these diseases. For example, efforts to genetically alter the ability of mosquitoes to transmit pathogens would be greatly facilitated by understanding the role of genes that determine vector competence and pathogen transmission (4–7).
One method of determining protein function of specific genes is to inhibit gene expression and observe the biological consequence. Gene function in Drosophila melanogaster often is analyzed by transposon-mediated insertional inactivation of the appropriate gene in a transgenic organism (8). Transformation of the Aedes aegypti mosquito genome recently has been described (9, 10), but germ-line transformation remains a laborious and time-consuming procedure for characterization, mutagenesis, and expression of genes in mosquitoes. Additionally, the complex life cycles of a number of medically important mosquitoes make routine transgenesis difficult.
Transient expression systems may more easily and rapidly answer biological questions posed by researchers. Virus transducing systems that efficiently express genes of interest (GOIs) in mosquitoes offer great potential for gene characterization. The double subgenomic Sindbis (dsSIN) viruses, based on the mosquito-borne virus Sindbis (SIN; Alphavirus; Togaviridae), allow long-term, stable, cytoplasmic expression of GOIs in mosquitoes (4, 11–13). SIN viruses are enveloped mosquito-borne RNA viruses that are approximately 70 nm in diameter (14). SIN virus replication and morphogenesis has been reviewed (14). dsSIN viruses are derived from fully infectious cDNA clones of SIN viruses and have been genetically engineered to express heterologous gene sequences in infected cells (13, 15, 16). These engineered viruses differ from naturally occurring SIN viruses because they contain a second subgenomic RNA promoter element immediately to the 3′ end of the structural genes of the virus, which transcribes a second subgenomic mRNA of a GOI or a nontranslatable effector RNA sequence. dsSIN viruses transcribe three mRNA species (genomic, first subgenomic, and second subgenomic mRNAs) in infected cells (Fig. 1), resulting in abundant expression of the effector RNAs; dsSIN viruses can generate up to 105 effector RNAs/cell in infected mosquito cells by 96 hr postinfection (17).
Figure 1.
(A) pTE/3′2J/anti-luc linearized by digestion with XhoI. A 595-base fragment from the 5′ end of the Luc gene was ligated into the multiple cloning site behind the second subgenomic promoter in antisense orientation. (B) The three predicted species of viral RNA transcripts expressed in mosquito cells. Viral nonstructural proteins are translated directly from genomic RNA. Structural protein mRNA is transcribed from the negative-sense strand at the subgenomic promoter; the heterolous antisense LUC segment also is transcribed. Antisense LUC mRNA also is transcribed from the negative-sense strand at the second subgenomic promoter. NS, nonstructural; S, structural; NCR, noncoding region.
dsSIN viruses already have been engineered that infect the salivary glands of Ae. aegypti and efficiently express effector RNAs complementary to specific gene sequences within the genomes of yellow fever and dengue-2 viruses (Flaviviridae) (18, 19). Specific effector RNAs have been identified that profoundly inhibit the replication of yellow fever and dengue-2 viruses in salivary glands of Ae. aegypti, which prevents transmission of these viruses. Antisense RNA interference with the replication of these viruses is presumed to function by hybridization between viral mRNA and the antisense RNA by Watson–Crick base pairing (20–22). Gene expression may be inhibited by activation of sequence-specific ribonucleases induced by double-stranded RNA within the cell (23–25). Posttranscriptional inhibition of endogenous gene expression also has been demonstrated in eukaryotes, including plants (26), Drosophila (27, 28), and Caenorhabditis elegans (24). Plant RNA viruses have been used to inhibit expression of green fluorescent protein transiently expressed in the nucleus of host plants (29).
To determine whether dsSIN viruses could be exploited to target mRNAs transcribed from mosquito genes, we used a transgenic line of Ae. aegypti that expresses a quantifiable reporter gene (9). This line was developed by using a binary Hermes transposable element system derived from Musca domestica (9, 30, 31) to transform a white-eye mutant (khw) of Ae. aegypti (32). The Hermes donor plasmid contained a phenotypic marker gene, cinnabar, derived from Drosophila melanogaster (33), and a reporter gene, luciferase (luc), regulated by the Ae. aegypti Apyrase (Apy) promoter (10). Apyrase is a 5′ nucleotidase produced in the salivary glands of female mosquitoes, which inhibits vertebrate host platelet aggregation during blood feeding (34–36). In the transgenic mosquitoes, luciferase (LUC) is expressed in the same developmental- and tissue-specific manner as the endogenous Apy gene in wild-type Ae. aegypti (10, 34), predominantly in the distal-lateral and medial lobes of the salivary glands of female adult mosquitoes. A dsSIN virus, designated TE/3′2J/anti-luc (Anti-luc), was engineered to express a 595-base RNA sequence complementary to the 5′ end of the LUC mRNA. We report here the sequence-specific inhibition of LUC protein production in transgenic mosquito salivary glands through expression of antisense RNA.
Materials and Methods
Construction of Anti-luc and Control dsSIN Viruses.
The construction of pTE/3′2J has been described (15, 16). pGEM-luc (Promega) was digested with EcoRI and BamHI, and a 595-bp fragment (position 1162–1757) was ligated into the EcoRI/BamHI site of pBluescript II SK (Stratagene). pBSIISK/5′luc was digested with HindIII, and the ends were filled in with the Klenow fragment of DNA polymerase I (E. coli) and religated to create an NheI site (19). pBSIISK/NheI/5′luc then was digested with XbaI and NheI. The fragment was subcloned into the XbaI site of pTE/3′2J, producing pTE/3′2J/anti-luc (Fig. 1A). The antisense orientation of the insert was confirmed by restriction endonuclease digestion and PCR using primers derived from the virus and insert sequence.
Suppression of gene expression by sense or antisense RNA in both plant and animal cells depends on a high level of sequence identity between the effector and target RNAs (18, 37). To observe whether inhibition of LUC activity by dsSIN virus was sequence specific, we included a nonhomologous antisense RNA as the control virus. A dsSIN virus, TE/3′2J/anti-D1GDD, which expresses a 240-base sequence complementary to the dengue-1 virus nonstructural protein 5 at the GDD motif (position 9399–9641 of the dengue-1 virus genome), was used as a control virus.
Generation of dsSIN Viruses.
Recombinant virus was produced from pTE/3′2J by linearization with XhoI, transcription from the SP6 promoter, and electroporation into BHK-21 cells (38). Tissue culture 50% infective dose (TCID50) of the virus, measured as log10 per ml, was determined by titration in triplicate in baby hamster kidney 21 (BHK-21) cells. Viruses with TCID50 of 8.2–8.5 log10/ml were used for inoculation in these experiments.
Mosquito Rearing.
The generation and characterization of the transgenic Ae. aegypti pH[cn]APY(1.6)LUC, line #43 (APY-LUC43), has been described (10). APY-LUC43 generation 8 (G8) mosquitoes were obtained as eggs; G12 adults were used in these experiments. Adult APY-LUC43 mosquitoes were maintained during the course of the experiments at 28°C and 80% humidity, with 12-hr light/dark cycles, and sugar and water provided ad libitum. In experiments requiring blood-fed mosquitoes, the preparation of blood and presentation of the blood meal was performed as described (39).
Intrathoracic Inoculation of Mosquitoes.
Adult female mosquitoes were cold-anesthetized and intrathoracically injected by using a Drummond 100-μl microcapillary needle that had been prepared with a needle puller (Narishige, Tokyo). Approximately 1 μl of dsSIN virus (≅105 infectious particles) in L-15 medium containing 10% FBS and 1% antibiotics was injected into each mosquito (19, 40).
Indirect Immunofluorescence Assay.
Salivary glands from each treatment group were dissected in water, mounted on acid-washed slides in a droplet of diluted Elmer’s glue, dried, and fixed in acetone for 10 min at −20°C (16). Infection of the salivary glands with dsSIN virus was confirmed by indirect immunofluorescence assay using a primary antibody that recognizes Sindbis E1 antigen diluted 1:200 (41) and visualized on an Olympus BH-2 epifluorescence microscope.
LUC Activity Assay.
Salivary glands were dissected in 0.15 M NaCl buffer and stored at −70°C in 30 μl of lysis buffer (Promega). Salivary glands were thawed at room temperature, sonicated in an Aquasonics waterbath (model 75S, VWR Scientific) for 2 min at 50°C, power level 5, then centrifuged (Hermle Z233M, Wehningen, Germany) at 13,000 rpm briefly. LUC assays were performed according to the manufacturer’s directions (Promega). Briefly, 20 μl of each sample was added to 100 μl of LUC assay reagent. LUC activity was measured as relative light units (RLU) by using a Turner TD-20e luminometer (Promega).
Apyrase Activity Assay.
Apyrase activity was determined by using the protocol of Ribeiro et al. (35) and an inorganic phosphorus commercial kit (Sigma). Salivary glands were dissected in 0.15 M NaCl and transferred to 100 μl of 10 mM Tris⋅Cl (pH 7.5). Salivary glands were homogenized in an Aquasonics waterbath (VWR Scientific) for 2 min at 50°C, power level 5, then centrifuged at 13,000 rpm for 1 min. Five microliters of salivary gland homogenate was added to 95 μl of reaction medium (100 mM Tris⋅HCl buffer (pH 9.0), 200 mM NaCl, 10 mM CaCl2, and 20 mM ADP sodium salt) and incubated at 37°C for 30 min. The reaction was stopped by adding 25 μl of ammonium molybdate in 2.5 N sulfuric acid (Sigma). The amount of inorganic phosphate released was visualized by adding 3 μl of Fiske & Subbarow reducing reagent (Sigma) and determined by using a 450 Plate Reader (Bio-Rad) at 665 nm. Apyrase activity was calculated as the total amount of monophosphate released per microgram of salivary gland protein.
Total Protein Concentration.
Salivary gland samples assayed for LUC and apyrase activity also were assayed for protein concentration by using the Pierce-Coomassie Plus Protein assay reagent in a 96-well plate according to the manufacturer’s instructions. Protein concentration was determined by using a 450 Plate Reader at 595 nm compared with BSA standards.
Western Blot Assay.
Salivary glands were dissected in 0.15 M NaCl then transferred to a tube containing 12 μl of nonreducing loading buffer. Homogenization consisted of three cycles of freeze-thawing and boiling for 2 min. One complete salivary gland was loaded per lane and proteins were separated on a 10% SDS/PAGE 1-mm gel (NOVEX, Hercules, CA) run at 150 mV for 1 hr. Proteins were transferred to a nitrocellulose membrane (Trans-blot, Bio-Rad) in 10% methanol transfer buffer at 200 mA for 3 hr. Proteins were detected by using the ECL-Western blotting kit (Amersham Pharmacia) according to the manufacturer’s instructions, except that PBS-0.05% NP-40 was used for blotting and all washes. A polyclonal rabbit anti-apyrase antibody raised to recombinant apyrase peptide diluted 1:20,000 detected the 68-kDa apyrase protein (42). The membrane was stripped and reprobed with a polyclonal rabbit anti-D7 antibody diluted 1:2,000, which detected the 37-kDa D7 protein (43). Amersham anti-rabbit horseradish peroxidase secondary antibody, diluted 1:1000, was used as secondary antibody. The membrane was exposed to Kodak X-Omat AR (VWR Scientific) x-ray film for 2 sec for both detections. Total salivary gland protein was detected by rinsing the membrane twice in PBS-0.05% NP-40 then incubating with 50 ml of colloidal gold (Bio-Rad) for 1 hr.
Virus Assays of Infected Mosquitoes.
Virus titrations were performed to confirm virus infection. Heads and abdomens in L-15 diluent were triturated with a pestle then passed through a 2-μm pore-size filter, as described (16). Virus titers were determined by end point dilution assay in triplicate in Vero cells (44).
Statistical Analysis.
Because of the variation of LUC protein expression among individual mosquitoes within a treatment group, the SDs were larger, in some cases, than the mean. To normalize the data points so that treatment groups could be compared, ANOVA and contrast statements of the sample values were calculated from log10 of RLU values, by using the sas 6.12 statistical program.
Results
Anti-luc Virus Characterization.
Transcription of the three viral mRNA species of Anti-luc virus (Fig. 1) was confirmed by Northern blot analysis of infected Aedes albopictus C6/36 cells (data not shown) (19). Mosquito infection was confirmed by determining viral titers in carcasses (Table 1). Mean titers ranged from 4.0 to 5.6 log10 TCID50 per mosquito. In addition, SIN antigen was detected in all lobes of the salivary glands of mosquitoes infected with Anti-luc and control virus at 2 and 10 days postinoculation (Fig. 2 B and C). This finding confirmed that the dsSIN viruses infect the same tissue of the salivary gland that expresses LUC in the transgenic mosquitoes, specifically the distal-lateral and medial lobes (10).
Table 1.
LUC activity in salivary glands of transgenic (APY-LUC43) mosquitoes
| n (% survival) | Average TCID50 (log10/ml) | Mean (SE) RLU/μg protein | P value* | |
|---|---|---|---|---|
| 5 days postinoculation | ||||
| Uninoculated | 20 (78) | NI | 272 (142) | |
| Control virus | 19 (73) | 5.6 | 210 (125) | |
| Anti-luc virus | 20 (78) | 5.2 | 18 (9) | 0.001 |
| 9 days postinoculation | ||||
| Uninoculated | 17 (83) | NI | 21 (4) | |
| Control virus | 14 (73) | 4.5 | 14 (4) | |
| Anti-luc virus | 16 (70) | 4.0 | 3 (1) | 0.0001 |
NI, not infected.
LUC activity (RLU/μg protein) in Anti-luc virus-infected vs. uninfected and control virus-infected mosquitoes as calculated by contrast statement.
Figure 2.
Indirect immunofluorescence assay showing APY-LUC43 female mosquito salivary glands (A) 10 days after intrathoracic inoculation with saline, (B) 48 hr after inoculation with Anti-luc virus, and (C) 10 days after inoculation with Anti-luc virus. SIN virus infection is confirmed in B and C by use of a primary antibody that recognizes SIN E1 protein (41). DL, distal-lateral lobe; M, medial lobe; PL, proximal-lateral lobe. Magnifications: A, ×200; B and C, ×100.
LUC Expression in APY-LUC43 Mosquitoes Infected with Anti-luc Virus.
LUC activity was assayed in dissected salivary glands of experimental mosquitoes. Female mosquitoes that had eclosed less than 24 hr earlier were cold-anesthetized and intrathoracially injected with Anti-luc virus or control virus. Uninoculated controls were cold-anesthetized only. Salivary glands were collected at 5 and 9 days postinoculation. The 9-day group was blood-fed on day 6.
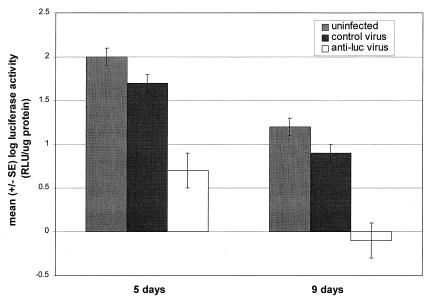
LUC activity in dissected salivary glands was measured and standardized to the total protein of the salivary gland sample (average protein concentration per salivary gland: 2.2 μg in uninfected, 2.6 μg in control virus, and 1.8 μg in Anti-luc virus-infected mosquitoes). Mean LUC activity at 5 days postinoculation was 272 RLU/μg protein in uninoculated controls and 210 RLU/μg protein in mosquitoes infected with control virus, compared with 18 RLU/μg protein in mosquitoes infected with Anti-luc virus (Table 1). Mean LUC activity at 9 days was 21, 14, and 3 RLU/μg protein for uninfected, control virus-infected, and Anti-luc virus-infected mosquitoes, respectively. Data were transformed to log10 and analyzed for evidence of LUC inhibition. LUC activity in Anti-luc-infected mosquitoes was reduced >90% compared with uninfected controls at both 5 (0.7 vs. 2.0 log10 RLU/μg protein; P = 0.0001) and 9 days (−0.7 vs. 1.2 log10 RLU/μg protein; P = 0.0001) postinfection (Fig. 3). Mean LUC activity in salivary glands of virus controls and uninfected controls was not significantly different at 5 days (1.7 vs. 2.0 log10 RLU/μg protein; P = 0.17) or 9 days (0.9 vs. 1.2 log10 RLU/μg protein; P = 0.2) postinfection (Fig. 3).
Figure 3.
Mean log10 LUC activity, measured as RLU standardized to total protein concentration of the salivary glands, in APY-LUC43 mosquitoes 5 and 9 days after intrathoracic injection with either Anti-luc virus or control virus. Uninoculated control mosquitoes were cold-anesthetized only.
Apyrase and Western Blot Assays.
Mean salivary gland apyrase activity did not differ statistically at 5 or 9 days postinfection between uninfected mosquitoes and those infected with Anti-luc virus (Table 2). Western blot assay showed comparable amounts of apyrase and D7 proteins in Anti-luc virus-infected and uninfected salivary glands (Fig. 4 A and B). In addition, total protein profiles and concentrations did not differ between Anti-luc-infected and uninfected salivary glands (Fig. 4C).
Table 2.
Apyrase activity in salivary glands
| 5 day Mean ± SE | N | 9 day Mean ± SE | N | |
|---|---|---|---|---|
| Uninfected | 252 ± 61 | 6 | 252 ± 59 | 7 |
| Anti-luc virus | 182 ± 49 | 7 | 272 ± 57 | 5 |
Apyrase activity was measured as the amount of monophosphate produced per microgram of salivary gland protein (PO4/μg protein). The 9-day mosquitoes were blood-fed on day 6.
Figure 4.
Western blot assay showing (A) the 37-kDa D7 protein, (B) the 68-kDa apyrase protein, and (C) total protein stained from the nitrocellulose membrane. Lanes 1, one pair of uninfected salivary glands; lanes 2, one pair of salivary glands infected with Anti-luc virus for 5 days.
Discussion
LUC production in APY-LUC43 mosquitoes was significantly inhibited by Anti-luc virus infection at both 5 and 9 days. LUC activity from dissected salivary glands was measured and standardized to the total salivary gland protein to eliminate the effect variable salivary gland size might have on LUC expression. Expression was inhibited >10-fold compared with uninfected and virus controls at both 5 and 9 days postinfection (Table 1 and Fig. 3). The difference in LUC expression between mosquitoes that were either uninfected or infected with control virus was not statistically significant (Table 1 and Fig. 3). In a previous experiment using total thoraces, LUC expression in mosquitoes infected with control virus increased 2-fold over uninoculated controls at 5 (56 vs. 22 RLU) and 9 (51 vs. 21 RLU) days postinoculation. Although the reason for this difference is currently unknown, it is important to note that at no time did the control dsSIN virus depress LUC expression. There were no apparent pathogenic effects of infection by the dsSIN viruses, as mosquitoes infected with control virus had survival rates similar to uninfected controls (Table l), similar to previous observations (45).
In addition, enzyme activity and concentrations of other proteins produced in the same salivary gland tissue did not differ in Anti-luc virus-infected and uninfected mosquitoes. Apyrase activity was not significantly different between uninfected mosquitoes and those infected with Anti-luc virus (Table 2). Apyrase protein, as measured by Western blot assay, was abundant in both treatment groups (Fig. 4B). Similarly, D7, a female-specific salivary gland protein produced in the same tissue as apyrase and LUC, was present in equivalent amounts in both uninfected and Anti-luc-infected mosquitoes (Fig. 4A). Finally, the total protein profile and concentrations were also equivalent in the two groups (Fig. 4C). Thus, the dsSIN system would seem to be a useful tool for characterizing salivary gland genes.
The individual variability of LUC expression is a confounding factor in the experiments. LUC expression in uninoculated controls could vary by as much as 100-fold (data not shown). The APY-LUC43 line was derived from one male and 10 females, and some genetic variability could account for the wide range of LUC expression, which has been noted since establishment of the line (C.J.C. and A.A.J., unpublished data). Alternatively, the surrounding chromatin structure at the genomic site of insertion may influence Apy promoter activity. Environmental factors such as nutritional status and body size upon eclosion also could contribute to variability in apyrase promoter activity. We were unable to analyze the transcriptional activity of LUC, as LUC mRNA is not detectable by Northern blot analysis (10).
LUC expression was measured in individual mosquitoes. The majority of mosquitoes infected with Anti-luc virus had approximately 1% the LUC activity of controls. However, in a few Anti-luc virus-infected individuals, LUC activity approached control levels (data not shown), which resulted in a group mean of 10% LUC activity compared with controls. It is possible that the lack of inhibition of LUC expression seen in these few Anti-luc virus infected individuals is caused by less than 100% infection of the salivary gland cells expressing LUC. Once a cell is infected the amount of antisense RNA transcribed by the dsSIN virus is probably sufficient to inhibit LUC expression. dsSIN viruses have been shown to produce as many as 105 transcripts/cell in Aedes albopictus C6/36 cells (17), and LUC mRNA is undetectable in the transgenic mosquito salivary glands (10). Future experiments should test the ability of the dsSIN system to inhibit expression of more abundantly expressed endogenous mosquito genes.
Despite the individual variability of LUC expression and the few cases of breakthrough expression in mosquitoes infected with Anti-luc virus, LUC expression in APY-LUC43 mosquitoes was inhibited >90% by Anti-luc virus infection. Thus intrathoracic injection of mosquitoes with Anti-luc virus is an effective and specific method for inhibiting gene expression in vivo.
We have shown inhibition of a gene expressed from the mosquito genome using antisense RNA delivered by the dsSIN virus expression system. Works in progress include design of a dsSIN vector that can orally infect mosquitoes (46), which would reduce possible deleterious effects from the inoculation. Future targets for gene expression inhibition include the Apy gene itself, or an inducible endogenous gene, such as late trypsin in the midgut (47). dsSIN virus infection, and thus antisense transcript production, could be established before induction of the trypsin gene by blood feeding. Inhibition of gene expression in vivo will increase our knowledge of mosquito biology and further our understanding of the dynamic interactions between the pathogen and the mosquito vector.
Acknowledgments
We thank Drs. Thomas Keefe and William C. Black IV for their expertise and guidance in the statistical analyses. This work was supported by grants from the John D. and Catherine T. MacArthur Foundation and the National Institutes of Health (AI28781 and AI29746).
Abbreviations
- Anti-luc
TE/3′2J/anti-luc
- APY-LUC43
pH[cn]APY(1.6)LUC, line#43 transgenic Aedes aegypti
- dsSIN
double subgenomic Sindbis virus
- LUC (luc)
luciferase
- RLU
relative light units
- SIN
Sindbis
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gratz N. Annu Rev Entomol. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Division of Disease Prevention and Control. Epidemiol Bull Pan Am Health Org. 1997;18:1–6. [Google Scholar]
- 3.Robertson S E, Hull B P, Tomori O, Bele O, LeDuc J W, Esteves K. J Am Med Assoc. 1996;276:1157–1162. [PubMed] [Google Scholar]
- 4.Carlson J, Olson K, Higgs S, Beaty B. Annu Rev Entomol. 1995;40:359–388. doi: 10.1146/annurev.en.40.010195.002043. [DOI] [PubMed] [Google Scholar]
- 5.Crampton J M. Trans R Soc Trop Med Hyg. 1994;88:141–143. doi: 10.1016/0035-9203(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 6.Handler A M, O’Brochta D A. Annu Rev Entomol. 1991;36:159–183. doi: 10.1146/annurev.en.36.010191.001111. [DOI] [PubMed] [Google Scholar]
- 7.Curtis C. Parasitol Today. 1994;10:371–374. doi: 10.1016/0169-4758(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 8.Spradling A, Stern D, Kiss I, Roote J, Laverty T, Rubin G. Proc Natl Acad Sci USA. 1995;92:10824–10830. doi: 10.1073/pnas.92.24.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jasinskiene N, Coates C J, Benedict M Q, Cornel A J, Rafferty C S, James A A, Collins F H. Proc Natl Acad Sci USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coates C J, Jasinskiene N, Pott G B, James A A. Gene. 1999;226:317–325. doi: 10.1016/s0378-1119(98)00557-5. [DOI] [PubMed] [Google Scholar]
- 11.Higgs S, Powers A M, Olson K E. Parasitol Today. 1993;9:444–452. doi: 10.1016/0169-4758(93)90098-z. [DOI] [PubMed] [Google Scholar]
- 12.Olson K E, Higgs S, Hahn C, Rice C M, Carlson J O, Beaty B J. Insect Biochem Mol Biol. 1994;24:39–48. doi: 10.1016/0965-1748(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 13.Olson K, Beaty B, Higgs S. In: The Insect Viruses. Miller L K, Ball L A, editors. New York: Plenum; 1998. pp. 371–404. [Google Scholar]
- 14.Strauss J, Strauss E G. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn C S, Hahn Y S, Braciale T J, Rice C M. Proc Natl Acad Sci USA. 1992;89:2679–2683. doi: 10.1073/pnas.89.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgs S, Olson K, Kamrud K I, Powers A M, Beaty B J. In: Molecular Biology of Insect Disease Vectors: A Methods Manual. Crampton J, Beard C, Louis C, editors. London: Chapman & Hall; 1997. pp. 459–483. [Google Scholar]
- 17.Powers A M, Kamrud K I, Olson K E, Higgs S, Carlson J O, Beaty B J. Proc Natl Acad Sci USA. 1996;93:4187–4191. doi: 10.1073/pnas.93.9.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgs S, Rayner J O, Olson K E, Davis B S, Beaty B J, Blair C D. Am J Trop Med Hyg. 1998;58:663–670. doi: 10.4269/ajtmh.1998.58.663. [DOI] [PubMed] [Google Scholar]
- 19.Olson K E, Higgs S, Gaines P J, Powers A M, Davis B S, Kamrud K I, Carlson J O, Blair C D, Beaty B J. Science. 1996;272:884–886. doi: 10.1126/science.272.5263.884. [DOI] [PubMed] [Google Scholar]
- 20.Green P J, Pines O, Masayori I. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- 21.Coleman J, Green P J, Masayori I. Cell. 1984;37:429–436. doi: 10.1016/0092-8674(84)90373-8. [DOI] [PubMed] [Google Scholar]
- 22.Izant J G, Weintraub H. Science. 1985;229:345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- 23.Sharp P. Proc Natl Acad Sci USA. 1999;13:139–141. [Google Scholar]
- 24.Montgomery M K, SiQun X, Fire A. Proc Natl Acad Sci USA. 1998;95:15502–15507. doi: 10.1073/pnas.95.26.15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar M, Carmichael G G. Microbiol Mol Biol Rev. 1998;62:1415–1434. doi: 10.1128/mmbr.62.4.1415-1434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruening G. Proc Natl Acad Sci USA. 1998;95:13349–13351. doi: 10.1073/pnas.95.23.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennerdell J, Carthew R. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- 28.Misquitta L, Paterson B. Proc Natl Acad Sci USA. 1999;96:1451–1456. doi: 10.1073/pnas.96.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratcliff F G, MacFarlane S A, Baulcombe D C. Plant Cell. 1999;11:11207–1215. doi: 10.1105/tpc.11.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar A, Yardley K, Atkinson P W, James A A, O’Brochta D A. Insect Biochem Mol Biol. 1997;27:359–363. doi: 10.1016/s0965-1748(97)00018-0. [DOI] [PubMed] [Google Scholar]
- 31.Warren W D, Atkinson P W, O’Brochta D A. Genet Res Camberra. 1994;64:87–97. doi: 10.1017/s0016672300032699. [DOI] [PubMed] [Google Scholar]
- 32.Bhalla S C. Mosquito News. 1968;28:380–385. [Google Scholar]
- 33.Cornel A J, Benedict M Q, Rafferty C S, Howells A J, Collins F H. Insect Biochem Mol Biol. 1997;27:993–997. doi: 10.1016/s0965-1748(97)00084-2. [DOI] [PubMed] [Google Scholar]
- 34.Smartt C T, Kim A P, Grossman G L, James A A. Exp Parasitol. 1995;81:239–248. doi: 10.1006/expr.1995.1114. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro J M, Sarkis J J, Rossignol P A, Spielman A. Comp Biochem Physiol. 1984;79B:81–86. doi: 10.1016/0305-0491(84)90081-6. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro J M, Rossignol P, Spielman A. Insect Physiol. 1985;31:689–692. [Google Scholar]
- 37.Branch A D. Hepatology. 1996;24:1517–1529. doi: 10.1002/hep.510240634. [DOI] [PubMed] [Google Scholar]
- 38.Powers A M, Olson K E, Higgs S, Carlson J O, Beaty B J. Virus Res. 1994;32:57–67. doi: 10.1016/0168-1702(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 39.Higgs S, Beaty B J. In: The Biology of Disease Vectors. Beaty B J, Marquardt W C, editors. Niwot, CO: Univ. Press of Colorado; 1996. pp. 595–605. [Google Scholar]
- 40.Rosen L, Gubler D. Am J Trop Med Hyg. 1974;23:1153–1160. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- 41.Chanas A C, Ellis D S, Stamford S, Gould E A. Antiviral Res. 1982;2:191–201. doi: 10.1016/0166-3542(82)90042-0. [DOI] [PubMed] [Google Scholar]
- 42.Champagne D E, Smartt C T, Ribeiro J M, James A A. Proc Natl Acad Sci USA. 1995;92:694–698. doi: 10.1073/pnas.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James A A. Bull Institut Pasteur. 1994;92:133–150. [Google Scholar]
- 44.Karber G. Arch Exp Pathol Pharmacol. 1931;162:480–483. [Google Scholar]
- 45.Jupp P, Phillips J. African Entomol. 1998;6:75–81. [Google Scholar]
- 46.Seabaugh R C, Olson K E, Higgs S, Carlson J O, Beaty B J. Virology. 1998;243:99–112. doi: 10.1006/viro.1998.9034. [DOI] [PubMed] [Google Scholar]
- 47.Noriega F G, Barillas-Mury C, Wells M A. Insect Biochem Mol Biol. 1994;24:627–631. doi: 10.1016/0965-1748(94)90099-x. [DOI] [PubMed] [Google Scholar]