Abstract
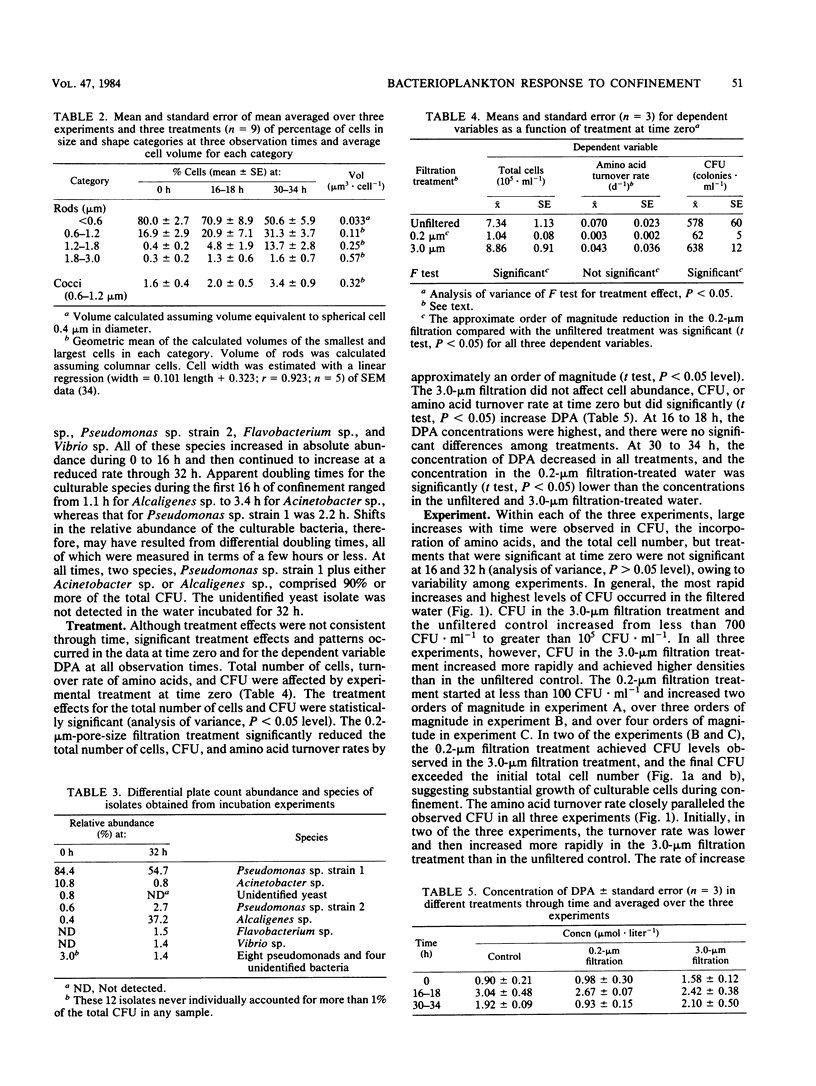
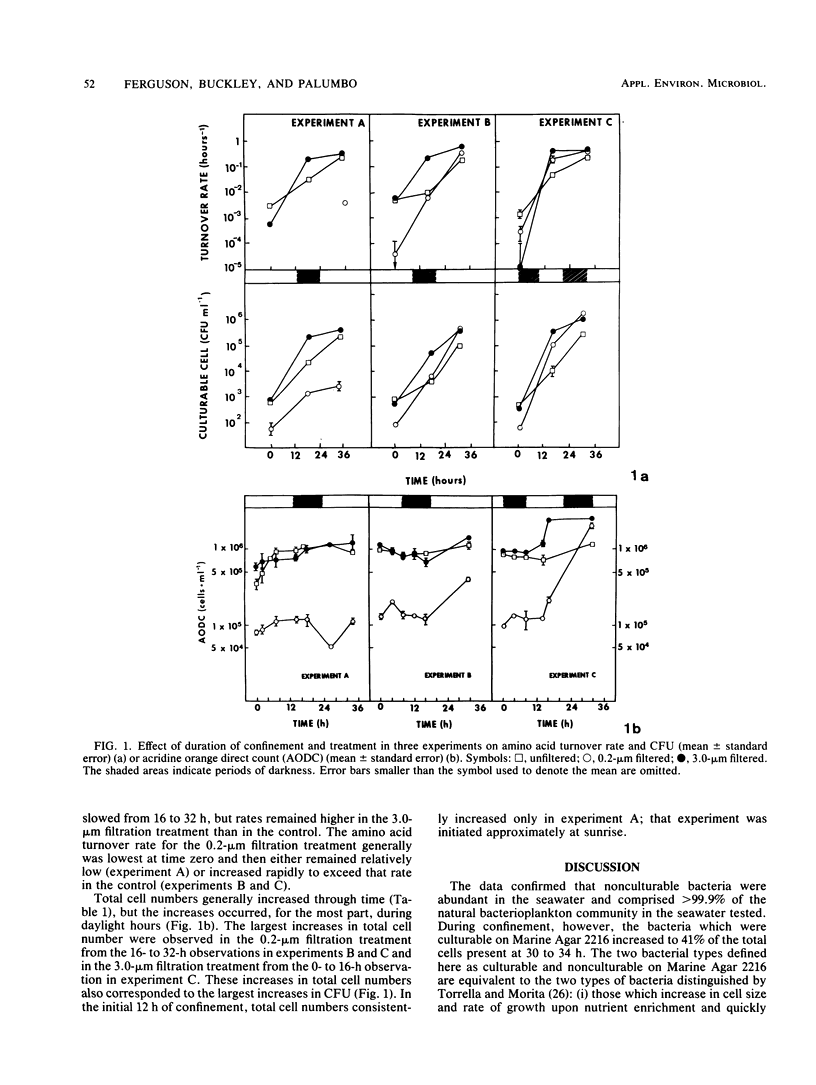
The bacterioplankton community of confined seawater at 25 degrees C changed significantly within 16 h of collection. Confinement increased CFU, total cell number (by epifluorescence microscopy), and average cell volume of bacterioplankton and increased the turnover rate of amino acids in seawater sampled at Frying Pan Shoals, N.C. The bacterioplankton community was characterized by two components: differential doubling times during confinement shifted dominance from bacteria which were nonculturable to bacteria which were culturable on a complex nutrient medium. Culturable cells (especially those of the genera Pseudomonas, Alcaligenes, and Acinetobacter) increased from 0.08% of the total cell number in the seawater immediately after collection to 13% at 16 h and 41% at 32 h of confinement. Differential filtration before confinement indicated that particles passing through a 3.9-microns-, but retained by a 0.2-micron-, pore-size Nuclepore filter may be a major source of primary amines to the confined population. The 3.0-microns filtration increased growth rate and ultimate numbers of culturable cells through the removal of bacterial predators or the release of primary amines from cells damaged during filtration or both.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Colwell R. R. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J Bacteriol. 1973 Jan;113(1):24–32. doi: 10.1128/jb.113.1.24-32.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Appl Environ Microbiol. 1976 Oct;32(4):617–622. doi: 10.1128/aem.32.4.617-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTTER L. F. The effect of pH on the development of bacteria in water strored in glass containers. Can J Microbiol. 1960 Jun;6:257–263. doi: 10.1139/m60-030. [DOI] [PubMed] [Google Scholar]
- Sieburth J. M., Willis P. J., Johnson K. M., Burney C. M., Lavoie D. M., Hinga K. R., Caron D. A., French F. W., 3rd, Johnson P. W., Davis P. G. Dissolved organic matter and heterotrophic microneuston in the surface microlayers of the north atlantic. Science. 1976 Dec 24;194(4272):1415–1418. doi: 10.1126/science.194.4272.1415. [DOI] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman S. A., Carey C. L. Decomposition of Organic Matter in Sea Water by Bacteria: I. Bacterial Multiplication in Stored Sea Water. J Bacteriol. 1935 May;29(5):531–543. doi: 10.1128/jb.29.5.531-543.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman S. A., Carey C. L. Decomposition of Organic Matter in Sea Water by Bacteria: II. Influence of Addition of Organic Substances upon Bacterial Activities. J Bacteriol. 1935 May;29(5):545–561. doi: 10.1128/jb.29.5.545-561.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. T. Measurement and significance of specific activity in the heterotrophic bacteria of natural waters. Appl Environ Microbiol. 1978 Aug;36(2):297–305. doi: 10.1128/aem.36.2.297-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]


