Abstract
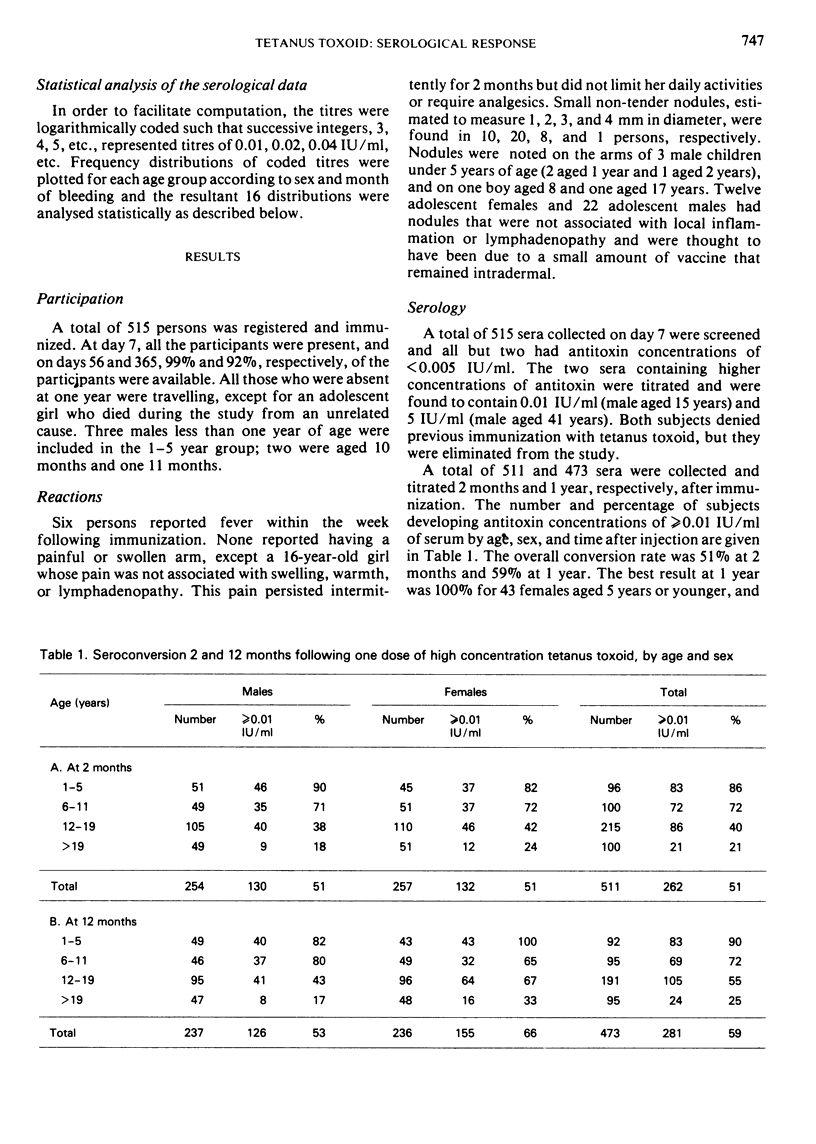
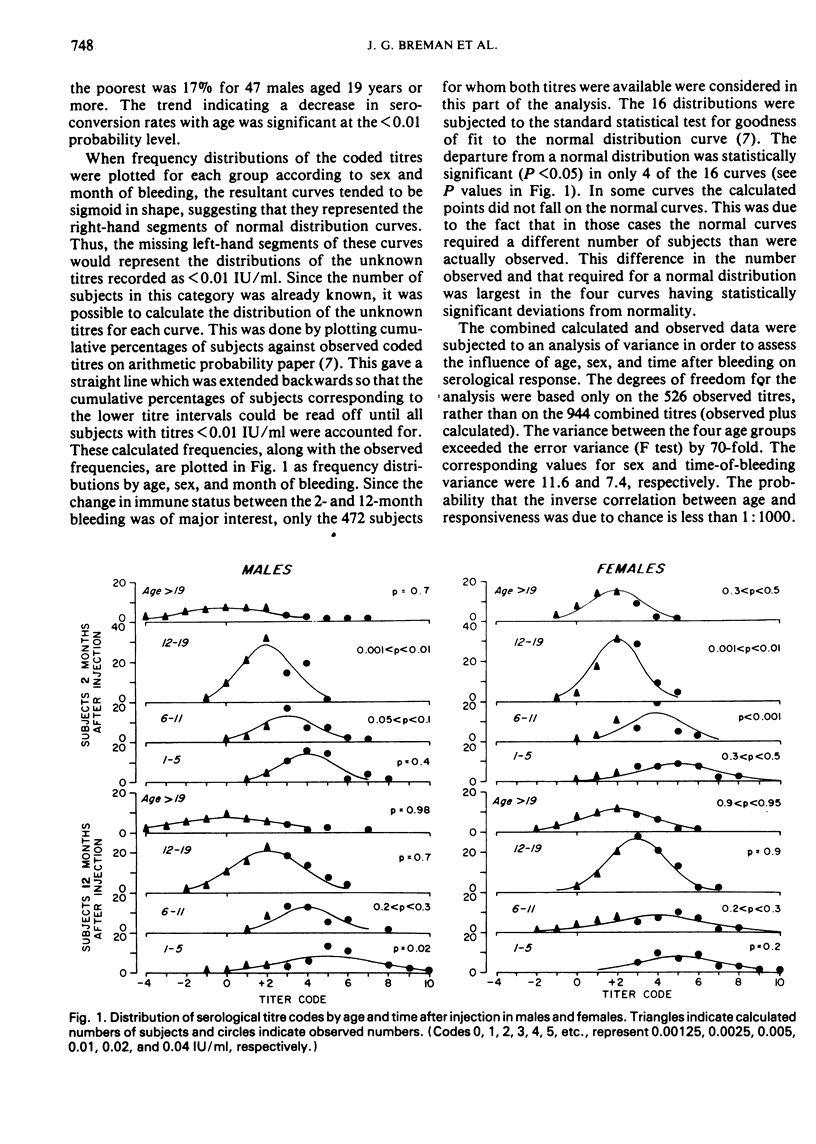
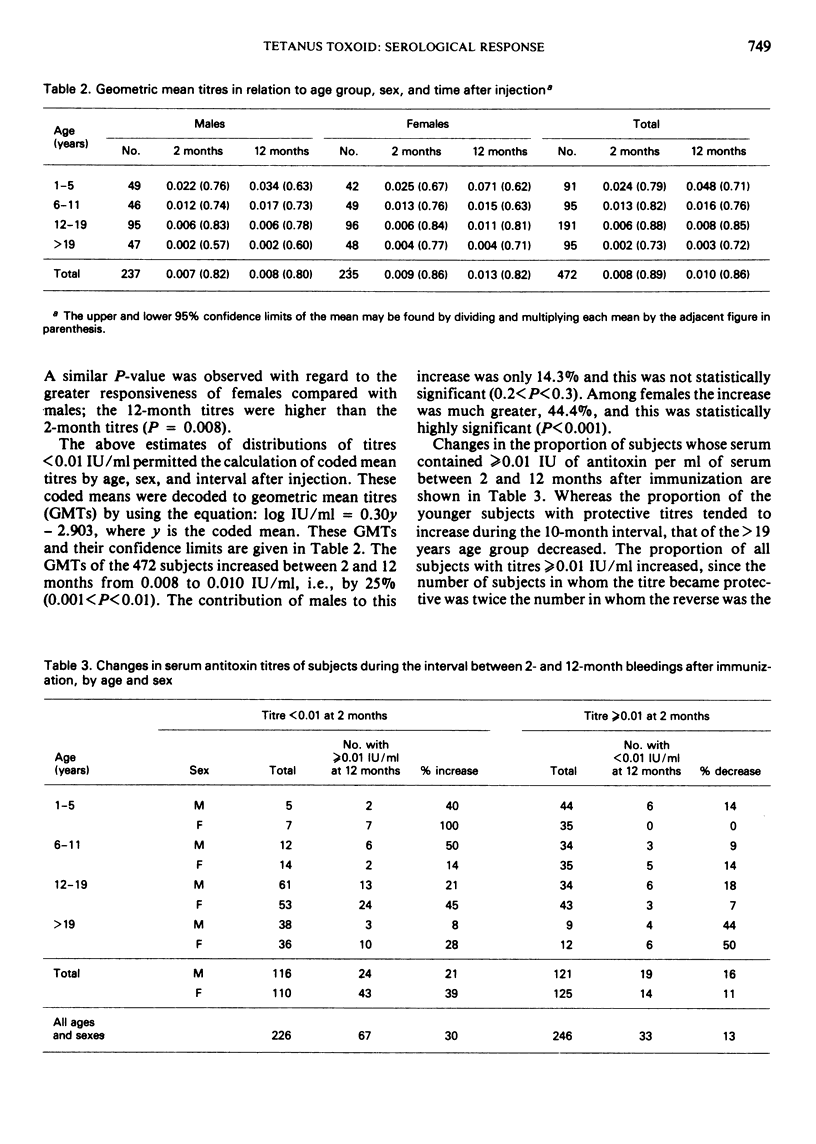
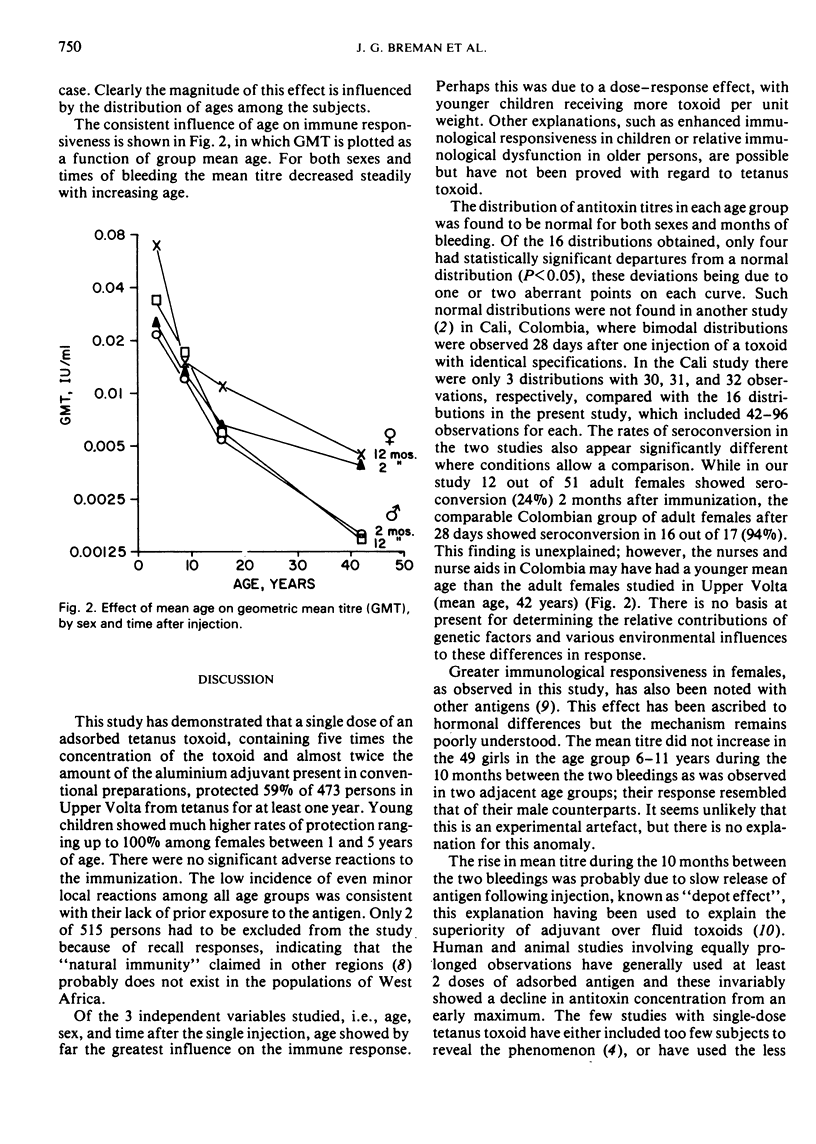
Single-dose immunization against tetanus was studied in 511 previously non-immunized residents of rural villages in Upper Volta. Males and females were equally represented and a wide age range was covered. A single dose of adsorbed tetanus toxoid containing 17.5 Lf units of toxoid and 3.86 mg of aluminium phosphate per 0.5 ml dose was used. Blood samples were taken 7 days, 2 months, and 12 months after immunization, and serum antitoxin titres were determined by neutralization titrations in mice. Adverse reactions were negligible. Only 2 participants gave evidence of prior immunization by developing detectable antitoxin titres after 7 days; they were eliminated from the study. After 12 months, 59% of the participants had antitoxin titres of ≥0.01 IU/ml, a titre usually considered protective. The mean titre and the proportion of those protected decreased substantially with increasing age; overall, females gave somewhat greater serological responses than males. Mean titre increased by 25% between 2 months and 1 year after immunization; the increase was greater in females than in males. In children under 6 years of age, 100% of females and 82% of males had protective titres after 1 year.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Durand B., Turpin A., Relyveld E. H., Carrie J., Robert D., Fontanges R., Rey M. Vaccination antitetanique simplifiee. Resultats preliminaires d'une etude Africaine. Dev Biol Stand. 1978;41:3–14. [PubMed] [Google Scholar]
- Edsall G., Belsey M. A., LeBlanc D. R., Levine L. Host factors in the response to immunization. Prog Drug Res. 1975;19:263–273. doi: 10.1007/978-3-0348-7090-0_30. [DOI] [PubMed] [Google Scholar]
- Maclennan R., Levine L., Newell K. W., Edsall G. The early primary immune response to absorbed tetanus toxoid in man: A study of the influence of antigen concentration, carrier concentration, and sequence of dosage on the rate, extent, and persistence of the immune response to one and to two doses of toxoid. Bull World Health Organ. 1973;49(6):615–626. [PMC free article] [PubMed] [Google Scholar]
- Newell K. W., Leblanc D. R., Edsall G., Levine L., Christensen H., Montouri M. H., Ramirez N. The serological assessment of a tetanus toxoid field trial. Bull World Health Organ. 1971;45(6):773–785. [PMC free article] [PubMed] [Google Scholar]
- Stanfield J. P., Gall D., Bracken P. M. Single-dose antenatal tetanus immunisation. Lancet. 1973 Feb 3;1(7797):215–219. doi: 10.1016/s0140-6736(73)90062-7. [DOI] [PubMed] [Google Scholar]
- Van Ramshorst J. D., Sundaresan T. K., Outschoorn A. S. International collaborative studies on potency assays of diphtheria and tetanus toxoids. Bull World Health Organ. 1972;46(2):263–276. [PMC free article] [PubMed] [Google Scholar]


