Abstract
Overactivity of the brain renin-angiotensin system (RAS) has been implicated in the development and maintenance of hypertension in several experimental models, such as spontaneously hypertensive rats and transgenic mice expressing both human renin and human angiotensinogen transgenes. We recently reported that, in the murine brain, angiotensin II (AngII) is converted to angiotensin III (AngIII) by aminopeptidase A (APA), whereas AngIII is inactivated by aminopeptidase N (APN). If injected into cerebral ventricles (ICV), AngII and AngIII cause similar pressor responses. Because AngII is metabolized in vivo into AngIII, the exact nature of the active peptide is not precisely determined. Here we report that, in rats, ICV injection of the selective APA inhibitor EC33 [(S)-3-amino-4-mercaptobutyl sulfonic acid] blocked the pressor response of exogenous AngII, suggesting that the conversion of AngII to AngIII is required to increase blood pressure (BP). Furthermore, ICV injection, but not i.v. injection, of EC33 alone caused a dose-dependent decrease in BP by blocking the formation of brain but not systemic AngIII. This is corroborated by the fact that the selective APN inhibitor, PC18 (2-amino-4-methylsulfonyl butane thiol), administered alone via the ICV route, increases BP. This pressor response was blocked by prior treatment with the angiotensin type 1 (AT1) receptor antagonist, losartan, showing that blocking the action of APN on AngIII metabolism leads to an increase in endogenous AngIII levels, resulting in BP increase, through interaction with AT1 receptors. These data demonstrate that AngIII is a major effector peptide of the brain RAS, exerting tonic stimulatory control over BP. Thus, APA, the enzyme responsible for the formation of brain AngIII, represents a potential central therapeutic target that justifies the development of APA inhibitors as central antihypertensive agents.
Keywords: brain, renin-angiotensin system, zinc metalloproteases, mercapto inhibitors, blood pressure
One-fifth of the adult population suffers from chronic hypertension resulting in significant morbidity and mortality. Most (95%) have essential hypertension, whose etiology remains uncertain. The implication of a central component in animal models and in humans has been suggested, which could be at the origin of the sympathetic hyperactivity observed at early stages of this pathology (1, 2). Overactivity of the brain renin-angiotensin system (RAS) has been implicated in the development and maintenance of hypertension in several types of experimental and genetic hypertension models, such as spontaneously hypertensive rats (SHR) (3–5), transgenic rats harboring the mouse renin Ren 2d gene (6, 7), and transgenic mice expressing both human renin and human angiotensinogen transgenes (8). All components of the RAS, including the precursor and enzymes required for the production and degradation of angiotensins (Angs) and Ang receptors, have been identified in the brain. Among the main bioactive peptides of the brain RAS, Ang-(1–8) (AngII) and its direct metabolite, Ang-(2–8) (AngIII), have the same affinity for type 1 (AT1) and type 2 (AT2) AngII receptors (for reviews, see refs. 9–11). The intracerebroventricular (ICV) injection of AngII or AngIII causes dose-dependent pressor responses that involve sympathetic activation, synaptic inhibition of the baroreflex at the level of the nucleus of the tractus solitarius, and the release of vasopressin (9, 12, 13). However, because AngII may be converted to AngIII in vivo, the exact nature of the active peptide is not determined precisely.
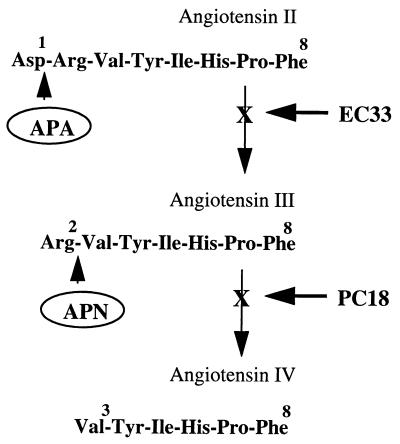
We recently reported that in the murine brain, aminopeptidase A (APA, EC 3.4.11.7), a membrane-bound zinc-metallopeptidase (14–17), hydrolyzes in vivo the N-terminal aspartate of AngII to generate AngIII. In contrast, aminopeptidase N (APN, EC 3.4.11.2), another zinc-metallopeptidase (18, 19) from the same family, the gluzincins (20), hydrolyzes the N-terminal arginine of AngIII to generate angiotensin IV (21, 22) (Fig. 1). In an attempt to define the respective roles of brain AngII and AngIII in the central control of cardiovascular functions, we recently have developed highly selective APA and APN inhibitors: the compound EC33 [(S)-3-amino-4-mercaptobutyl sulfonic acid] specifically inhibits APA whereas the compounds EC27 [(S)-2-amino-pentan-1,5-dithiol] and PC18 (2-amino-4-methylsulfonyl butane thiol) specifically inhibit APN (22–24). Using these new tools, we demonstrated previously that AngIII and not AngII, as shown at the periphery, is one of the main effector peptides of the brain RAS in the central control of vasopressin release and supraoptic vasopressinergic neuron activity (21, 22, 25).
Figure 1.
Metabolic pathways of AngII and AngIII in the brain involving zinc-metallopeptidases. EC33, APA inhibitor; PC18, APN inhibitor.
This prompted us to delineate the respective roles of AngII and AngIII in the central control of arterial blood pressure (BP) by blocking each of their metabolic pathways with selective APA and APN inhibitors, respectively. If brain AngIII proves to be the active peptide of the brain RAS in the control of BP, this study will allow, in addition, to demonstrate the efficacy of APA inhibitors as central antihypertensive agents in an experimental hypertension model, the SHR rat.
Materials and Methods
Drugs.
EC33 and PC18 were synthesized by the laboratory of B. P. Roques (Institut National de la Santé et de la Recherche Médicale, Unité 266; and Centre National de la Recherche Scientifique, UMR 8600) as described previously (23, 24). Human AngII and human AngIII were purchased from Sigma. The AT1 receptor antagonist losartan was obtained from DuPont, and the AT2 receptor antagonist PD123319 was purchased from Research Biochemicals (Natick, MA).
Animals.
Experiments were performed on mature normotensive Wistar Kyoto (WKY) rats and SHR weighing 300–350 g. These animals were obtained from Iffa Credo (L’Arbresle, France) and kept under artificial light (12-h light/12-h dark cycle) with a normal standard diet (Usine alimentation Rationnelle; Epinay-sur-Orge, France) and water given ad libitum. All animal procedures were conducted in agreement with the guiding principles for the care and use of animals approved by the Society for Neuroscience.
Intracerebroventricular Injections in Anesthetized Rats.
All compounds injected ICV or i.v. were dissolved in sterile 0.9% saline, and the pH was adjusted to 7.0 with 0.1 M NaOH. Mature male SHR and WKY rats were anesthetized with 100 mg/kg i.p. Inactin [5-ethyl-2-(1′methylpropyl)-2-thiobarbiturate Research Biochemicals] and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). A 26-gauge stainless steel guide cannula was implanted just above the roof of the right lateral ventricle (stereotaxic coordinates with respect to bregma: 1-mm caudal and 1.5-mm lateral; ref. 26) and was lowered 4 mm below the surface of the skull. The guide cannula was anchored to the skull by using acrylic dental cement. Peptides and inhibitors were injected by inserting a 33-gauge stainless steel internal cannula into the guide cannula so that it extended 1 mm beyond the tip of the guide into the lateral ventricle. This injector was connected to a 10-μl Hamilton syringe via polyethylene (PE20) tubing. A left femoral artery catheter (PE50) filled with heparinized saline (250 units/ml) also was inserted for recording arterial BP.
Intracerebroventricular and Intravenous Injections into Alert Rats.
The male SHR used for conscious experiments were anesthetized with pentobarbital sodium (50 mg/kg i.p.; Sentravet Laboratory, Plancoët, France) and assigned to two groups. In the first, a guide cannula was positioned stereotaxically in the lateral ventricle, and in the second, an additional catheter was introduced into the femoral vein. A femoral artery catheter was inserted into all animals as described above. The femoral arterial and venous catheters were brought under the skin and emerged at the nape of the neck. Flexible, metal springs were attached to the skull and neck of the rat and connected to dual-channel swivels mounted directly above the cage. This arrangement allowed the rat free movement within the cage. Each rat then was given an intramuscular injection of 0.1 ml of penicillin-streptomycin (50,000 units/ml; Boehringer Mannheim) and allowed to recover for at least 24 h before the experiment. The animals were not allowed access to food or water during the experiments.
Blood Pressure Recording.
Mean arterial BP was recorded continuously throughout each experiment by using a COBE CDX III pressure transducer (Phymep, Paris, France) connected to the MacLab system (Phymep) consisting of a MacLab hardware unit and chart software running on a Macintosh computer. Heart rate was triggered by the BP signal.
At the end of each experiment, the correct placement of the lateral cerebroventricular cannula was checked by the postmortem ICV injection of 5 μl of Fast Green dye (Sigma) by using the same injector system as was used for each experiment. The brain was then removed to check that the dye was present in the cerebroventricular system.
Data and Statistical Analysis.
The maximal change from baseline BP induced by the injection of drugs into the lateral ventricle was calculated for each animal, for each dose of each drug tested. Values are given as mean ± SEM. Results were analyzed by ANOVA, followed by Student’s paired t test (before and after ICV injection) and unpaired t test (WKY rats vs. SHR). Differences with P < 0.05 were considered significant.
Results
Effect of the ICV Injection of the APA Inhibitor EC33 on the Angiotensin-Induced BP Increase in Anesthetized WKY Rats and SHR.
First, we tested our model by monitoring BP changes after a single ICV injection of AngII or AngIII in anesthetized WKY rats and SHR. Rats were anesthetized with Inactin, a compound currently used for renal physiology experiments, which gave BP responses similar to those in the alert condition (27). As expected, there were significant differences in mean ± SEM baseline BP between normotensive WKY rats (117.9 ± 1.4 mmHg; 1 mmHg = 133 Pa) and SHR (172.5 ± 2.5 mmHg). There was no significant difference in baseline mean arterial BP (MABP) between the members of each group before injection. We first checked that ICV injection of saline did not affect baseline blood pressure. Changes in MABP (ΔMABP) were induced by 10–100 ng of AngII or AngIII within 30 s of injection in anesthetized WKY rats and SHR. In WKY rats, similar increases in MABP were observed after injection of 10 ng of AngII (ΔMABP = +9.0 ± 0.6 mmHg; duration = 7.9 ± 0.7 min, n = 9) or 30 ng of AngII (ΔMABP = +8.8 ± 0.8 mmHg; duration = 7.8 ± 0.6 min, n = 5), whereas after injection of AngIII, a comparable increase in BP was obtained for a dose of 30 ng (ΔMABP = +10.5 ± 0.8 mm Hg; duration = 8.2 ± 0.6 min, n = 10) rather than for a dose of 10 ng (ΔMABP = +7.6 ± 0.9 mmHg; duration = 6.4 ± 0.9 min, n = 5). The maximum change obtained between 60 and 100 ng (ΔMABP = +13 mmHg) was routinely recorded after 1–2 min, as described previously (13), and BP values returned to baseline levels within 10–15 min. The same observations were obtained in SHR (not shown). So, to evaluate the effect of EC33 on equivalent AngII- or AngIII-induced BP increases, we have chosen the dose of 10 ng for AngII and 30 ng for AngIII.
WKY rats then were injected ICV with saline or EC33, and, 15 min later, an ICV injection of AngII and AngIII was given. We found that ICV injection of AngII (10 ng) significantly increased BP (ΔMABP = +9.0 ± 0.6 mmHg), which returned to baseline 7.9 min after the start of the injection (Table 1). The AngII-induced pressor response was abolished completely in the presence of the selective APA inhibitor, EC33, at the dose of 100 μg. A similar pressor effect (+10.5 ± 0.8 mmHg) was obtained after ICV injection of AngIII (30 ng) alone, and this effect lasted 8.2 ± 0.6 min. In contrast to the abolition of the AngII-induced pressor effect observed in the presence of EC33, the prior treatment with this APA inhibitor (100 μg) did not change the pressor response induced by AngIII (30 ng) (ΔMABP = +7.3 ± 1.4 mmHg). Similar experiments were done in anesthetized SHR (Table 1). We found that ICV injection of AngII (10 ng) increased significantly BP (ΔMABP = +11.3 ± 1.2 mmHg), which returned to baseline 11.6 ± 0.4 min after the start of the injection. The AngII-induced pressor response was completely abolished in the presence of the selective APA inhibitor, EC33, at the dose of 100 μg. A similar pressor effect (+11.1 ± 0.6 mmHg) was obtained after ICV injection of AngIII (30 ng) alone, and this effect lasted 9.8 ± 1.0 min. In contrast to the abolition of the AngII-induced pressor effect observed in the presence of EC33, the prior treatment with this APA inhibitor (100 μg) did not significantly change the pressor response induced by AngIII (30 ng) (Δ MABP = +7.5 ± 2.2 mmHg).
Table 1.
Effect of ICV injection of the APA inhibitor EC33 on angiotensin-induced BP increase in anesthetized normotensive WKY rats and SHR
| Rats | Drugs | ΔMABP, mmHg | Duration, min |
|---|---|---|---|
| WKY | Saline + AngII (10 ng), n = 9 | 9.0 ± 0.6* | 7.9 ± 0.7* |
| EC33 (100 μg + AngII (10 ng), n = 6 | 0.7 ± 0.6NS | 1.3 ± 1.0NS | |
| Saline + AngIII (30 ng), n = 10 | 10.5 ± 0.8* | 8.2 ± 0.6* | |
| EC33 (100 μg) + AngIII (30 ng), n = 8 | 7.3 ± 1.4*† | 11.5 ± 2.4*† | |
| SHR | Saline + AngII (10 ng), n = 12 | 11.3 ± 1.2* | 11.6 ± 0.4* |
| EC33 (100 μg) + Ang II (10 ng), n = 5 | 1.25 ± 0.7NS | 1.0 ± 0.6NS | |
| Saline + AngIII (30 ng), n = 14 | 11.1 ± 0.6* | 9.8 ± 1.0* | |
| EC33 (100 μg) + AngIII (30 ng), n = 6 | 7.5 ± 2.2*† | 12.5 ± 3.8*† |
Anesthetized normotensive WKY rats and SHR were given (ICV) saline or EC33, followed 15 min later by an ICV injection of AngII or AngIII. The mean ± SEM baseline BP 14 min after ICV injection of saline was 118.9 ± 2.6 mmHg and 116.9 ± 1.8 mmHg for AngII and AngIII, respectively, and the mean BP 14 min after the ICV injection of EC33 was 90.5 ± 2.9 mmHg and 101.0 ± 4.9 mmHg for AngII and AngIII, respectively, for WKY rats. The mean ± SEM baseline BP 14 min after ICV injection of saline was 163.4 ± 4.0 mmHg and 165.7 ± 3.8 mmHg for AngII and AngIII, respectively, and the mean BP 14 min after the ICV injection of EC33 was 148.5 ± 2.2 mmHg and 149.5 ± 5.3 mmHg for AngII and AngIII, respectively, for SHR. ΔMABP after AngII or AngIII injection were determined and compared with prepeptide injection BP levels. Mean ± SEM time required for a return to prepeptide injection BP levels also is shown. n, number of rats individually analyzed. *, P < 0.05. NS, Nonsignificant as compared with prepeptide injection BP levels.
†Nonsignificant as compared with the ΔMABP induced by AngIII alone.
The pressor effect of AngII (10 ng) in the absence or presence of EC33 (100 μg) also was studied in conscious SHR. As in the anesthetized animals, we observed that the prior treatment with the APA inhibitor completely abolished the AngII-induced pressor response (AngII + EC33 ΔMABP = +1.3 ± 0.7 mmHg, duration = 1.6 ± 0.8 min, n = 4 vs. AngII alone ΔMABP = +22.0 ± 2.6 mmHg, duration = 10.3 ± 2.6 min, n = 4) (data not shown).
ΔMABP in Anesthetized and Alert WKY Rats and SHR After ICV or Intravenous Injection of the APA Inhibitor Alone.
Anesthetized rats.
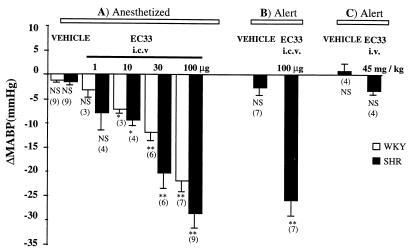
The inhibition of endogenous AngIII formation by the ICV injection of EC33 (1–100 μg) alone decreased BP in a dose-dependent manner in anesthetized WKY rats and SHR (Figs. 2A and 4B). The maximal effect of EC33, at the dose of 100 μg, was significantly greater in SHR (ΔMABP = −28.7 ± 2.9 mmHg) than in WKY rats (ΔMABP = −22.0 ± 2.1 mmHg; P < 0.05). At this dose, BP did not return to baseline until 4 h after EC33 injection in WKY rats and SHR.
Figure 2.
Mean arterial blood pressure changes in WKY rats and SHR after ICV or i.v. injection of APA inhibitor (EC33). Shown are mean ± SEM changes in arterial BP (ΔMABP in mmHg) after ICV injection of EC33 (1–100 μg) into anesthetized WKY rats and SHR (A), into alert SHR (B), or after i.v. injection of EC33 (45 mg/kg) into alert SHR (C). Mean ± SEM baseline blood pressure was 114.5 ± 1.5 mmHg for anesthetized WKY rats, 170.0 ± 1.9 mmHg for anesthetized SHR, and 151.5 ± 2.8 mmHg for alert SHR after ICV injection of saline and 157.5 ± 3.8 mmHg after i.v. injection of saline into alert SHR. The numbers in brackets indicate the number of animals. Mean ± SEM arterial blood pressure values obtained after EC33 injection were compared with baseline mean blood pressure obtained after saline injection. ∗, P < 0.05; ∗∗, P < 0.001.
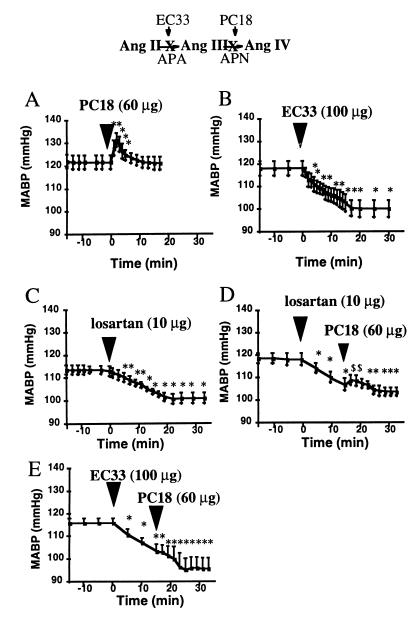
Figure 4.
Effects of the APA inhibitor (EC33) and AT1 receptor antagonist (losartan) on the APN inhibitor (PC18)-induced increase in arterial blood pressure in anesthetized normotensive WKY rats. The rats were assigned to one of five groups. Those in the first, second, and third groups were given an ICV injection of saline followed 15 min later by an ICV injection of PC18 (A), EC33 (B), or losartan (C). The fourth group first was given losartan (D), and the fifth was given EC33 (E) followed 15 min later by an ICV injection of PC18. Mean arterial blood pressure (mean ± SEM) of each of the five groups was monitored for 1 h. There were significant changes (∗, P < 0.05) in mean BP after the ICV injection of PC18 (A), EC33 (B), and losartan (C) compared with baseline mean BP obtained after saline injection. A significant increase ($, P < 0.05) in mean arterial BP was obtained after ICV injection of PC18 (D) over the mean BP after losartan pretreatment. (E) ICV injection of PC18 after EC33 pretreatment did not significantly increase mean BP.
Alert rats.
The results concerning the ICV or i.v. injection of the APA inhibitor, EC33, in alert SHR are presented in Fig. 2 B and C. Central injection of EC33 (100 μg) alone induced a hypotensive effect (ΔMABP = −26.0 ± 3.1 mmHg) in conscious, unrestrained SHR similar in amplitude to that observed in anesthetized SHR (Fig. 2B), but its duration was shorter (40.0 ± 8.5 min). In contrast, EC33 given by i.v. bolus administration, at a dose of 45 mg/kg, did not affect BP in alert SHR (Fig. 2C).
Effect of ICV Injection of the APN Inhibitor (PC18) on Angiotensin III-Induced BP Increase in Anesthetized WKY Rats and SHR.
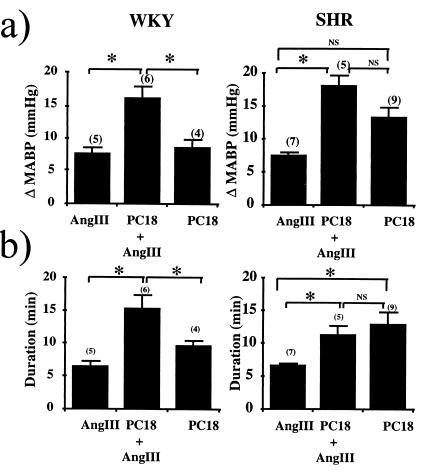
The ICV injection of AngIII (10 ng) alone induced similar pressor responses in anesthetized WKY rats (ΔMABP = +7.6 ± 0.9 mmHg; duration = 6.4 ± 0.9 min) and SHR (ΔMABP = +7.4 ± 0.5 mmHg; duration = 6.6 ± 0.3 min) (Fig. 3). If the APN inhibitor PC18 was injected alone via the ICV route into anesthetized WKY rats, a maximal pressor response was observed for doses of 60–100 μg (Figs. 3 and 4A). In WKY rats, PC18 (100 μg) induced a pressor effect similar in amplitude and duration to that obtained with AngIII (10 ng) alone. In contrast, in SHR, the amplitude and duration of the PC18 (60 μg) induced pressor effect were greater than those for the pressor response after injection of AngIII (10 ng) alone, probably because of the increase in endogenous angiotensin levels observed in the brain of this strain of animals as compared with normotensive WKY rats (28, 29) (Fig. 3). If AngIII (10 ng) was coinjected with PC18 (100 μg), an additivity of the pressor effects of AngIII and PC18 was observed in WKY rats. In contrast, in SHR, this additivity was not observed although the pressor response induced by AngIII (10 ng) with PC18 (60 μg) was greater than that obtained for the response to AngIII (10 ng) alone but was not significantly different from that obtained for the response to PC18 (60 μg) alone (Fig. 3). This pointed to the predominant participation of endogenous AngIII in the maximal pressor response in SHR obtained after injection of AngIII with PC18.
Figure 3.
Effects of the APN inhibitor PC18 on the AngIII-induced BP increase in anesthetized WKY rats and SHR. Mean ± SEM baseline BP was 180.0 ± 7.1 mmHg and 112.5 ± 1.7 mmHg for anesthetized SHR and WKY rats, respectively. Anesthetized WKY rats and SHR were given (ICV) AngIII (10 ng), PC18 (100 μg in WKY rats; 60 μg in SHR) or AngIII (10 ng) + PC18 (100 μg in WKY rats; 60 μg in SHR). (a) ΔMABP after the ICV injection of AngIII with PC18 were measured and compared with the ΔMABP obtained after the ICV injection of AngIII alone or PC18 alone. The numbers in parentheses are the number of animals; ∗, P < 0.05. (b) Mean ± SEM time required for a return to prepeptide injection BP levels. Mean ± SEM ΔMABP duration after the ICV injection of AngIII with PC18 was determined and compared with the ΔMABP duration obtained after the ICV injection of AngIII alone or PC18 alone. n = number of rats individually analyzed; ∗, P < 0.05.
Effects of the APA Inhibitor (EC33) and the AT1 Receptor Antagonist (losartan) on the APN Inhibitor (PC18)-Induced Increase in Arterial BP in Anesthetized WKY Rats and SHR.
PC18, given as an ICV injection, at a dose of 60 μg induced an elevation in BP (ΔMABP = +10.5 ± 0.5 mmHg; duration = 6.5 ± 1.5 min) in anesthetized WKY rats (Fig. 4A). In WKY rats, 80% of the PC18-induced increase in BP was blocked by a 15-min pretreatment with the AT1 antagonist losartan (10 μg ICV) (Fig. 4D). In these conditions, the maximal MABP change was +2.0 ± 1.2 mmHg and the duration of the effect was 2.4 ± 1.1 min. The ICV injection of losartan alone at the same dose induced by itself a significant hypotensive effect (Fig. 4C). The maximal change (ΔMABP = −9.8 ± 0.3 mmHg) was observed 20 min after the injection. In contrast, the PC18-induced increase in BP was not modified by a 15-min pretreatment with the AT2 antagonist PD 123319 (10 μg ICV) (ΔMABP PC18 + PD123319 = +9.3 ± 3.6 mmHg; duration = 9.7 ± 3.7 min, n = 4; data not shown). In SHR (data not shown), the pressor effect induced by 60 μg of PC18 (ΔMABP = +12. 1 ± 1.8 mmHg; duration = 13.6 ± 1.7 min, n = 9) was reduced only by 50% by ICV pretreatment with 10 μg of losartan (ΔMABP = +6.0 ± 1.8 mmHg; duration = 3.4 ± 0.9 min, n = 7). The ICV injection of losartan alone in SHR at the same dose induced by itself a significant hypotensive effect (ΔMABP = −18.6 ± 3.4 mmHg, n = 7). The involvement of central but not peripheral AT1 receptors is shown by the fact that i.v. bolus administration of 10 μg of losartan does not suppress the BP increase induced by 60 μg of ICV PC18 (ΔMABP = +11.0 ± 1.5 mmHg; duration = 9.0 ± 2.1 min, n = 4). The pretreatment with the APA inhibitor EC33 (100 μg), by inhibiting the formation of endogenous AngIII, blocked completely the PC18-induced increase in BP (Fig. 4E).
Discussion
The present study using selective APA and APN inhibitors demonstrates that (i) AngIII is the main effector peptide of the brain RAS, as compared with AngII, in regulating BP, (ii) brain AngIII exerts a tonic stimulatory action on the control of BP, (iii) APA inhibitors, administered centrally, by blocking the formation of brain AngIII, induce antihypertensive effects, more marked in spontaneously hypertensive than in normotensive rats.
As shown previously (21, 22), APA and APN, two ectoenzymes, were involved in brain in the metabolism of AngII and AngIII, respectively (Fig. 1). Wright and colleagues (30) were the first to investigate the respective roles of AngII and AngIII by blocking their metabolic pathways using aminopeptidase inhibitors such as bestatin (31) and amastatin (32). They showed that ICV treatment with amastatin or bestatin induced robust pressor responses in alert rats, which could be blocked by ICV treatment with the angiotensin receptor antagonist (Sar1, Thr8) AngII (sarthran). However, amastatin and bestatin are nonselective aminopeptidase inhibitors (33), and their effects in vivo are the result of a combined inhibitory effect on APA, APN, and other aminopeptidases. Therefore, we designed specific and selective APA and APN inhibitors: the APA inhibitor EC33 exhibits an inhibitory potency nearly 100-fold better for APA (Ki = 0.29 μM) than for APN (Ki = 25 μM) (24), whereas the inhibitory potency of the APN inhibitor PC18 was 2,150- and 125-fold more active on APN (Ki = 0.008 ± 0.001 μM) than on APA (Ki = 17.2 ± 4.3 μM) and aminopeptidase B (APB, EC 3.4.11.6) (Ki = 1 ± 0.2 μM) (22, 23), another monozinc aminopeptidase, cleaving preferentially N-terminal basic residues of peptides (34, 35). Injected by ICV route, these compounds in mice, by blocking, respectively, APA and APN, increased the half-life of brain AngII and AngIII by 2.3- and 3.9-fold (21, 22). Therefore, we used in vivo these potent inhibitors either to block in the brain the conversion of AngII to AngIII or to prevent AngIII degradation to delineate the respective roles of brain AngII and AngIII in the central control of BP in normotensive WKY rats or SHR. The SHR is an animal model of human essential hypertension that exhibits an overactive brain RAS, which has been implicated in the elevated expression and maintenance of high BP in these animals when compared with its normotensive control, namely the WKY rats (3, 9, 36).
In WKY rats as well as in SHR, central application of low doses of AngII and AngIII has been shown to induce equivalent and transient increases in BP (37). The brain structures involved in the modulation of BP by angiotensins are located in the forebrain (subfornical organ, organum vasculosum of the lamina terminalis, median preoptic nucleus, paraventricular and supraoptic nuclei) and in the lower brainstem (nucleus of the tractus solitarius, area postrema, dorsal motor nucleus of vagus, nucleus ambiguus, ventrolateral medulla). Most of these nuclei are rich in AT1 receptors and in angiotensinergic nerve terminals or cell bodies (38–40).
The present study showed that central pretreatment with the APA inhibitor EC33 blocked the pressor response of ICV AngII in anesthetized normotensive or spontaneously hypertensive rats as well as in conscious spontaneously hypertensive rats, suggesting that the conversion of AngII to AngIII is a prerequisite to its action on BP. That the same dose of EC33 was ineffective on the increase in BP produced by ICV AngIII demonstrates the specificity of action of the APA inhibitor on AngII metabolism. Interestingly, the blockade of endogenous brain AngIII formation by the ICV injection of EC33 alone induced an intense decrease in BP in a dose-dependent manner in anesthetized WKY rats and SHR, with a maximal effect more marked in SHR than in WKY rats. A similar effect was observed in alert SHR after ICV but not i.v. injection of EC33. Altogether, these data show that the central but not peripheral conversion of AngII to AngIII is required to increase BP and that endogenous brain AngIII and not AngII, as shown at the periphery, exerts a tonic, stimulatory action on the central control of BP at least in conscious SHR.
Although no specific APA or APN inhibitors were available until recently to show directly the importance of AngIII, the concept that AngII is not the main centrally active form of angiotensins in the central control of BP has emerged gradually from several studies. Ang receptors in brain membranes initially were thought to have a higher affinity for AngIII than for AngII (41). However, in the brain, AngIII subsequently was shown to have a pressor effect nearly as potent as that of AngII (42) and to be more potent than AngII when applied iontophoretically to paraventricular neurons to increase their firing rate (43). Two reports strengthen our findings by showing that ICV infusion of APA produces a significant increase in BP (44) and that ICV injection of APA antiserum reduces the increase in BP caused by exogenous AngII (45). There are many possible sites of action of APA inhibitors, because APA has been visualized in circumventricular organs and also in nuclei involved in the control of BP, such as the paraventricular nucleus and the nucleus of the tractus solitarius (46, 47), all containing angiotensinergic nerve terminals and receptors. Furthermore, the greater decrease in BP in SHR after ICV injection of the APA inhibitor could be related to the greater APA activity observed in the brain nuclei of this strain rather than in normotensive WKY rats, suggesting a participation of APA in the RAS hyperactivity of the SHR brain (47).
To ensure that AngIII was really an endogenous effector peptide of the brain RAS on the central control of BP, we conducted another series of experiments on anesthetized WKY rats and SHR, using PC18, a highly potent and selective APN inhibitor. First, the coadministration of PC18 with a low dose of AngIII significantly increases the pressor response induced by AngIII, suggesting that the inhibition of exogenous AngIII degradation by APN is responsible for this effect. Second, that PC18 injected ICV alone is able to significantly increase BP indicates that the inhibition of the degradation of endogenous AngIII by APN leads to its accumulation and produces an increase in BP. These data are in agreement with the decrease in BP, occurring after the ICV infusion of APN in SHR (44), probably because of an increase AngIII metabolism. Third, this is supported by the finding that EC33 completely blocked the PC18-induced increase in BP, confirming that endogenous AngIII is produced from endogenous AngII under the action of APA. Last, in normotensive WKY rats, 80% of the PC18-induced increase in BP was blocked by prior injection of the AT1 antagonist losartan (but not by the AT2 antagonist PD 123319). This demonstrates further the specificity of action of APN on AngIII metabolism and the involvement of AngIII in the PC18-induced increase in BP: by blocking the action of APN, PC18 causes an accumulation of endogenous brain AngIII, which, in turn, results in an increase in BP via the activation of AT1 but not AT2 receptors. The involvement of central but not peripheral AT1 receptors was demonstrated further by the effects of i.v. bolus administration of losartan, which did not abolish the BP increase induced by the ICV injection of PC18. These results clearly demonstrate that AngIII is the major effector peptide of the brain RAS, exerting tonic stimulatory central control over BP.
The effects of the ICV injection of selective subtype antagonists suggest that the AT1 receptor subtype is involved in the AngIII-induced pressor response. This conclusion is consistent with numerous studies using angiotensin receptor antagonists and AT1 receptor antisense showing that the classic functions of the brain RAS are mediated by AT1 receptors (11, 48). However, these data raise the question as to why, if AngII and AngIII have similar affinities for the AT1 receptor, the conversion of brain AngII to AngIII is required to increase BP? Two hypotheses may be put forward.
(i) There may be another unknown, AT1-like specific receptor for AngIII, with a pharmacological profile similar to that of the AT1 receptor. This may explain why losartan, even at high doses, unlike EC33, did not completely prevent the PC18-induced increase in BP in WKY rats and even more in SHR. However, the greater effect of PC18 in SHR than in WKY rats and its higher resistance to losartan may be due to the effects of APN in vivo on neuropeptides other than AngIII, as documented previously (49, 50).
(ii) Blocking the conversion of AngII to AngIII by APA inhibitor treatment may favor the activation of other metabolic pathways inactivating AngII, as reported previously (51, 52), and producing in each case peptide fragments unable to interact with AT1 or AT2 receptors. In this hypothesis, in the absence of the APA inhibitor, AngIII produced from AngII under the action of APA could increase BP by interacting with AT1 receptors. This fits well with the absence of pressor response obtained after ICV injection of AngII (now via AngIII) in AT1A receptor-deficient mice (53).
Thus, we have demonstrated that (i) AngIII, generated by APA, is a major effector of the brain RAS, exerting a tonic central control over BP, at least in conscious SHR, and (ii) the inhibition of central, but not peripheral, APA with specific and selective inhibitors leads to a decrease in BP. Central APA, therefore, is interesting as a possible target for the treatment of hypertension. This suggests that specific and selective APA inhibitors should be developed that will cross the blood–brain barrier as putative, central, antihypertensive agents.
Acknowledgments
We thank Prof. C. Kordon, Dr. P. Meneton, and Dr. A. M. Nuyt for critical reading of the manuscript. The English text was edited by J. Knight. This research was supported by a grant from the Centre National de la Recherche Scientifique (Physique et Chimie du Vivant).
Abbreviations
- AngII
angiotensin II
- AngIII
angiotensin III
- AT1 and AT2
angiotensin receptor types 1 and 2, respectively
- APA
aminopeptidase A
- APN
aminopeptidase N
- ICV
intracerebroventricular
- RAS
renin-angiotensin system
- BP
blood pressure
- MABP
mean arterial BP
- ΔMABP
change in MABP
- SHR
spontaneously hypertensive rats
- WKY rats
Wistar Kyoto rats
References
- 1.Oparil S, Chen Y-F, Berecek K H, Calhoun D A, Wyss J M. In: Hypertension: Pathophysiology, Diagnosis, and Management. Laragh J H, Brenner B M, editors. New York: Raven; 1995. pp. 713–740. [Google Scholar]
- 2.Esler M. In: Hypertension: Pathophysiology, Diagnosis, and Management. Laragh J H, Brenner B M, editors. New York: Raven; 1995. pp. 755–773. [Google Scholar]
- 3.Ganten D, Hermann K, Bayer C, Unger T, Lang R E. Science. 1983;221:869–871. doi: 10.1126/science.6879184. [DOI] [PubMed] [Google Scholar]
- 4.Gutkind J S, Kurihara M, Castren E, Saavedra J M. J Hypertens. 1988;6:79–84. doi: 10.1097/00004872-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Tamura K, Umemura S, Nyui N, Yamakawa T, Yamaguchi S, Ishigami T, Tanaka S, Tanimoto K, Takagi N, Sekihara H, et al. Hypertension. 1996;27:1216–1223. doi: 10.1161/01.hyp.27.6.1216. [DOI] [PubMed] [Google Scholar]
- 6.Senanayake P D, Moriguchi A, Kumagai H, Ganten D, Ferrario C M, Brosnihan K B. Peptides. 1994;15:919–926. doi: 10.1016/0196-9781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 7.Averill D B, Matsumura K, Ganten D, Ferrario C M. Hypertension. 1996;27:591–597. doi: 10.1161/01.hyp.27.3.591. [DOI] [PubMed] [Google Scholar]
- 8.Davisson R, Y, G, Beltz T, Martin D, AK, J, Sigmund C D. Circulation Research. 1998;83:1047–1058. doi: 10.1161/01.res.83.10.1047. [DOI] [PubMed] [Google Scholar]
- 9.Phillips M I. Annu Rev Physiol. 1987;49:413–435. doi: 10.1146/annurev.ph.49.030187.002213. [DOI] [PubMed] [Google Scholar]
- 10.Saavedra J M. Endocr Rev. 1992;13:329–380. doi: 10.1210/edrv-13-2-329. [DOI] [PubMed] [Google Scholar]
- 11.Wright J W, Harding J W. Regul Pept. 1995;59:269–295. doi: 10.1016/0167-0115(95)00084-o. [DOI] [PubMed] [Google Scholar]
- 12.Saavedra J M. Endocr Rev. 1992;13:329–380. doi: 10.1210/edrv-13-2-329. [DOI] [PubMed] [Google Scholar]
- 13.Wright J W, Harding J W. Brain Res Rev. 1992;17:227–263. doi: 10.1016/0165-0173(92)90018-h. [DOI] [PubMed] [Google Scholar]
- 14.Wu Q, Lahti J M, Air G M, Burrows P D, Cooper M D. Proc Natl Acad Sci USA. 1990;87:993–997. doi: 10.1073/pnas.87.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanus D M, Engelstein D, Gastl G A, Gluck L, Vidal M J, Morrison M, Finstad C L, Bander N H, Albino A P. Proc Natl Acad Sci USA. 1993;90:7069–7073. doi: 10.1073/pnas.90.15.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Wang J, Cooper M D. Genomics. 1993;17:657–664. doi: 10.1006/geno.1993.1386. [DOI] [PubMed] [Google Scholar]
- 17.Vazeux G, Wang J, Cooper M, Corvol P, Llorens-Cortes C. J Biol Chem. 1996;271:9069–9074. doi: 10.1074/jbc.271.15.9069. [DOI] [PubMed] [Google Scholar]
- 18.Malfroy B, Kado-Fong H, Gros C, Giros B, Schwartz J C, Hellmiss R. Biochem Biophys Res Commun. 1989;161:236–241. doi: 10.1016/0006-291x(89)91586-6. [DOI] [PubMed] [Google Scholar]
- 19.Watt V M, Yip C C. J Biol Chem. 1989;264:5480–5487. [PubMed] [Google Scholar]
- 20.Hooper N M. FEBS Lett. 1994;354:1–6. doi: 10.1016/0014-5793(94)01079-x. [DOI] [PubMed] [Google Scholar]
- 21.Zini S, Fournie-Zaluski M C, Chauvel E, Roques B P, Corvol P, Llorens-Cortès C. Proc Natl Acad Sci USA. 1996;93:11968–11973. doi: 10.1073/pnas.93.21.11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reaux A, De Mota N, Zini S, Cadel S, Fournie-Zaluski M C, Roques B P, Corvol P, Llorens-Cortes C. Neuroendocrinology. 1999;69:370–376. doi: 10.1159/000054439. [DOI] [PubMed] [Google Scholar]
- 23.Fournié-Zaluski M C, Coric P, Turcaud S, Lucas E, Noble F, Maldonado R, Roques B P. J Med Chem. 1992;35:1259–1266. doi: 10.1021/jm00085a013. [DOI] [PubMed] [Google Scholar]
- 24.Chauvel E N, Coric P, Llorens-Cortès C, Wilk S, Roques B P, Fournié-Zaluski M C. J Med Chem. 1994;37:1339–1346. doi: 10.1021/jm00035a014. [DOI] [PubMed] [Google Scholar]
- 25.Zini S, Demassey Y, Fournie-Zaluski M-C, Bischoff L, Corvol P, Llorens-Cortes C, Sanderson P. NeuroReport. 1998;9/5:120–124. doi: 10.1097/00001756-199803300-00011. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney, Australia: Academic; 1986. [Google Scholar]
- 27.Zou A P, Cowley A W., Jr Hypertension. 1997;29:194–198. doi: 10.1161/01.hyp.29.1.194. [DOI] [PubMed] [Google Scholar]
- 28.Phillips M I, Strnstrom B. Clin Exp Hypertens [A] 1984;6:1939–1942. doi: 10.3109/10641968409046105. [DOI] [PubMed] [Google Scholar]
- 29.Phillips M I, Kimura B. J Hypertens. 1988;6:607–612. doi: 10.1097/00004872-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Jensen L L, Harding J W, Wright J W. Brain Res. 1989;490:48–55. doi: 10.1016/0006-8993(89)90429-0. [DOI] [PubMed] [Google Scholar]
- 31.Umezawa H, Aoyagi T, Suda H, Hamada M, Takeuchi T. J Antibiot. 1975;29:97–99. doi: 10.7164/antibiotics.29.97. [DOI] [PubMed] [Google Scholar]
- 32.Aoyagi T, Tobe H, Kojima F, Hamada M, Takeuchi T, Umezawa H. J Antibiot. 1978;31:636–638. doi: 10.7164/antibiotics.31.636. [DOI] [PubMed] [Google Scholar]
- 33.Checler F. In: Methods in Neurotransmitter and Neuropeptide Research. Parvez S H, Naoi M, Nagatsu T, editors. Amsterdam: Elsevier Science; 1993. , Chap. 13, pp. 375–418. [Google Scholar]
- 34.Cadel S, Pierotti A R, Foulon T, Créminon C, Barré N, Segrétin D, Cohen P. Mol Cell Biol. 1995;110:149–160. doi: 10.1016/0303-7207(95)03529-g. [DOI] [PubMed] [Google Scholar]
- 35.Hopsu V K, Mäkinen K K, Glenner G G. Arch Biochem Biophys. 1966;114:557–566. doi: 10.1016/0003-9861(66)90380-8. [DOI] [PubMed] [Google Scholar]
- 36.Berecek K H, Kirk K A, Nagahama S, Oparil S. Am J Physiol. 1983;252:H796–H806. doi: 10.1152/ajpheart.1987.252.4.H796. [DOI] [PubMed] [Google Scholar]
- 37.Wright J W, Morseth S L, Abhold R H, Harding J W. Am J Physiol. 1985;249:R514–R521. doi: 10.1152/ajpregu.1985.249.5.R514. [DOI] [PubMed] [Google Scholar]
- 38.Mendelsohn F, Quirion R, Saavedra J, Aguilera G, Catt K. Proc Natl Acad Sci USA. 1984;81:1575–1579. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lind R W, Swanson L W, Ganten D. Neuroendocrinology. 1985;40:2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- 40.Lenkei Z, Palkovits M, Corvol P, Llorens Cortes C. Front Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 41.Bennet J P, Snyder S H. Eur J Pharmacol. 1980;67:11–25. doi: 10.1016/0014-2999(80)90003-5. [DOI] [PubMed] [Google Scholar]
- 42.Wright J W, Jensen L L, Roberts K A, Sardinia M F, Harding J W. Am J Physiol. 1989;257:R1551–R1557. doi: 10.1152/ajpregu.1989.257.6.R1551. [DOI] [PubMed] [Google Scholar]
- 43.Harding J W, Felix D. Brain Res. 1987;410:130–134. doi: 10.1016/s0006-8993(87)80033-1. [DOI] [PubMed] [Google Scholar]
- 44.Wright J W, Mizutani S, Murray C E, Amir H Z, Harding J W. J Hypertens. 1990;8:969–974. doi: 10.1097/00004872-199010000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Song L, Wilk S, Healy D P. Brain Res. 1997;744:1–6. doi: 10.1016/s0006-8993(96)00952-3. [DOI] [PubMed] [Google Scholar]
- 46.Healy D P, Wilk S. Brain Res. 1993;606:295–303. doi: 10.1016/0006-8993(93)90997-2. [DOI] [PubMed] [Google Scholar]
- 47.Zini S, Masdehors P, Lenkei Z, Fournie-Zaluski M C, Roques B P, Corvol P, Llorens-Cortes C. Neuroscience. 1997;78:1178–1193. doi: 10.1016/s0306-4522(96)00660-4. [DOI] [PubMed] [Google Scholar]
- 48.Wright J W, Harding J W. Brain Res Brain Res Rev. 1997;25:96–124. doi: 10.1016/s0165-0173(97)00019-2. [DOI] [PubMed] [Google Scholar]
- 49.Waksman G, Boubouton R, Devin R, Bourgoin S, Cesselin F, Hamon M, Fournié-Zaluski M-C, Roques B P. Eur J Pharmacol. 1985;117:233–243. doi: 10.1016/0014-2999(85)90608-9. [DOI] [PubMed] [Google Scholar]
- 50.Llorens-Cortes C, Gros C, Schwartz J C. Proc Natl Acad Sci USA. 1986;83:6226–6230. doi: 10.1073/pnas.83.16.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrario C M, Chappell M C, Tallant E A, Brosnihan K B, Diz D I. Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 52.Chansel D, Czekalsi S, Vandermeersch S, Ruffet E, Fournie-Zaluski M C, Ardaillou R. Am J Physiol. 1998;275:F535–F542. doi: 10.1152/ajprenal.1998.275.4.F535. [DOI] [PubMed] [Google Scholar]
- 53.Davisson R L, Beltz T G, Oliverio M I, Johnson A K, Smithies O, Coffman T M, Sigmund C D. Hypertension. 1998;32:595. [Google Scholar]