Abstract
Recent work has shown that strychnine, the potent and selective antagonist of glycine receptors, is also an antagonist of nicotinic acetylcholine (AcCho) receptors including neuronal homomeric α7 receptors, and that mutating Leu-247 of the α7 nicotinic AcCho receptor-channel domain (L247Tα7; mut1) converts some nicotinic antagonists into agonists. Therefore, a study was made of the effects of strychnine on Xenopus oocytes expressing the chick wild-type α7 or L247Tα7 receptors. In these oocytes, strychnine itself did not elicit appreciable membrane currents but reduced the currents elicited by AcCho in a reversible and dose-dependent manner. In sharp contrast, in oocytes expressing L247Tα7 receptors with additional mutations at Cys-189 and Cys-190, in the extracellular N-terminal domain (L247T/C189–190Sα7; mut2), micromolar concentrations of strychnine elicited inward currents that were reversibly inhibited by the nicotinic receptor blocker α-bungarotoxin. Single-channel recordings showed that strychnine gated mut2-channels with two conductance levels, 56 pS and 42 pS, and with kinetic properties similar to AcCho-activated channels. We conclude that strychnine is a modulator, as well as an activator, of some homomeric nicotinic α7 receptors. After injecting oocytes with mixtures of cDNAs encoding mut1 and mut2 subunits, the expressed hybrid receptors were activated by strychnine, similar to the mut2, and had a high affinity to AcCho like the mut1. A pentameric symmetrical model yields the striking conclusion that two identical α7 subunits may be sufficient to determine the functional properties of α7 receptors.
Much evidence indicates that strychnine, a competitive antagonist of glycine receptors, is also a potent antagonist at nicotinic acetylcholine (AcCho) receptors (nAcChoRs). Thus, strychnine blocks reversibly and noncompetitively the embryonic type of muscle nAcChoRs and some forms of neuronal heteromeric nAcChoRs (1, 2). Moreover, strychnine antagonizes reversibly and competitively homomeric α7, α8, and α9 nAcChoRs expressed in Xenopus oocytes (3–7) and native α7-containing, α-bungarotoxin (α-BuTx)-sensitive nAcChoRs (2, 8).
The α7 nAcChoR is an α-BuTx-sensitive ligand gated ion channel exhibiting fast desensitization, nonlinear current-voltage (I–V) relation, and low affinity for AcCho. Mutating the α7 channel domain with a threonine-for-leucine substitution (L247Tα7; mut1), renders the receptor’s I–V relation linear, increases its affinity for AcCho, decreases desensitization, and converts curare, hexamethonium, 5-hydroxytryptamine, and dihydro-β-erythroidine (DHβE), from antagonists into agonists (9–11). Therefore, we thought it would be interesting to investigate whether strychnine behaves like the antagonists of wild-type α7 nAcChoR (WTα7) receptors that become agonists of L247Tα7 receptors, or whether, like fluoxetine (12), strychnine antagonizes both WTα7 and L247Tα7 receptors. We also were interested in studying α7 receptors with additional Ser for Cys mutations at positions 189 and 190 (L247T/C189–190Sα7; mut2), which correspond to Cys-192 and Cys-193 of Torpedo α1 nAcChoR and which are thought to be part of the AcCho binding site (reviewed in refs. 13–15).
Materials and Methods
Oocyte Injection.
Full-length cDNAs encoding the chick WTα7 or the mutated L247Tα7 neuronal nAcChoR subunits were kindly provided by M. Ballivet (University of Geneva, Geneva, Switzerland) and were expressed as described (9, 16, 17). Stage VI oocytes were injected intranuclearly with cDNA clones (2 ng cDNA in 10 nl of buffer). For coexpression experiments oocytes were injected with a mixture of 1 ng and 1 ng (ratio 1:1) or 5 ng cDNA (ratio 1:5), and control oocytes were injected with 1 or 5 ng cDNA, respectively.
Site-Directed Mutagenesis.
Mutagenesis was carried out by using the QuickChange site-directed mutagenesis system (Stratagene). The two point mutations responsible for cysteine substitution were induced on chick L247Tα7 by using HPLC-purified mutant oligonucleotides (5′-CTGAGAGCTTTTATGAGTCCTCTAAAGAACCATACCC-3′ and its complement). All mutations were confirmed by manual sequence analysis based on the Sanger dideoxy termination method (sequenase version 2.0 DNA sequencing kit, Amersham Pharmacia).
Electrophysiology.
Two to four days after injection, membrane currents were recorded in voltage-clamped oocytes by using two microelectrodes filled with 3 M KCl (18). The oocytes were placed in a recording chamber (volume, 0.1 ml) and perfused continuously, 10–11 ml/min, with oocyte Ringer’s solution (82.5 mM NaCl/2.5 mM KCl/2.5 mM CaCl2/2.5 mM MgCl2/5 mM Hepes/adjusted to pH 7.4 with NaOH) at room temperature (20–22°C). Unless otherwise specified, oocytes were voltage-clamped at −60 mV. To obtain dose/response relations AcCho was applied to the oocytes at 3-min intervals. The half-inhibitory concentration (IC50) of strychnine, as well as the concentration producing half-maximal effect (EC50) of AcCho/strychnine were estimated by fitting the data to Hill equations, by using least-square routines, detailed in refs. 11 and 12. The time for 10% decay (T0.1) of the current, that is, the time taken for the current to decay from its peak to 90%, was used to estimate the rate of receptor desensitization. I–V relationships were determined by using repetitive exposures to AcCho at various potentials, stepping the holding potential from −60 mV to the desired voltage 5–10 s before transmitter application; or using 2- to 3-s voltage ramps in the range of +40 mV to −140 mV, when currents were well maintained. When indicated, the oocytes were treated with α-BuTx (Sigma), diluted in oocyte Ringer’s solution containing BSA (100 μg/ml) to minimize adsorption of α-BuTx to the plastic surface.
Single-channel currents were recorded by using the patch-clamp technique in the outside-out configuration. AcCho and strychnine (Sigma) were dissolved in oocyte Ringer’s solution (35 μM and 10 μM, respectively) just before use. Borosilicate-glass patch pipettes (3–5 mΩ tip resistance) were filled with intracellular solution (80 mM CsF, 0.5 mM EGTA, 50 μM CaCl2, 5 mM Hepes, adjusted to pH 7.4 with CsOH). Drugs and washing solutions were applied to the excised patch membrane by a gravity-driven system using independent external pipettes for control Ringer’s solution and agonist-containing solutions, placed ≈100 μm from the recording pipette. Single-channel currents were recorded, filtered, sampled, and analyzed as in refs. 19 and 20.
Binding Assay on Oocytes.
Oocytes that had been injected with either mut2 or C189–190Sα7 mutant cDNA were electrophysiologically tested with 2 mM ACh and assayed for plasma-membrane α-BuTx binding following the procedures indicated (21).
Results
Strychnine Blocks WTα7 Receptors.
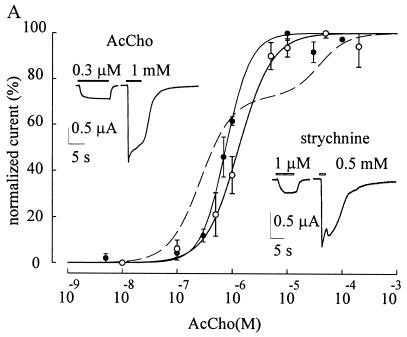
Oocytes injected with WTα7 subunit cDNA responded to AcCho (100 μM ≈ EC50; ref. 11) with a peak inward current (AcCho-activated current, IAcCho) of −448 ± 95 nA at −60 mV (mean ± SEM; range: −172 nA to −1.33 μA; 16 oocytes; three donors: 16/3). Strychnine alone (50 nM-3 mM) did not elicit obvious current responses in noninjected oocytes or oocytes expressing WTα7 nAcChoRs. In contrast, when strychnine was coapplied with AcCho the IAcCho peak amplitude was reduced without changing its decay, and the current recovered promptly after strychnine withdrawal (see Fig. 1A). The blockage of IAcCho increased as the strychnine concentration was raised, as illustrated in Fig. 1A, which also shows that the strychnine dose-IAcCho response relationship fitted well the Hill equation (11, 12) and gave IC50 and Hill number (nH) values of 1 μM and 1.7, respectively (6/3). In a number of experiments IAcCho elicited by 100 μM AcCho was fully suppressed by 5 μM strychnine. However, IAcCho peak amplitude was reduced only to 40% when the AcCho concentration was raised to 1 mM (3/1), supporting the view that strychnine acts as a competitive antagonist of AcCho at WTα7 nAcChoRs (2).
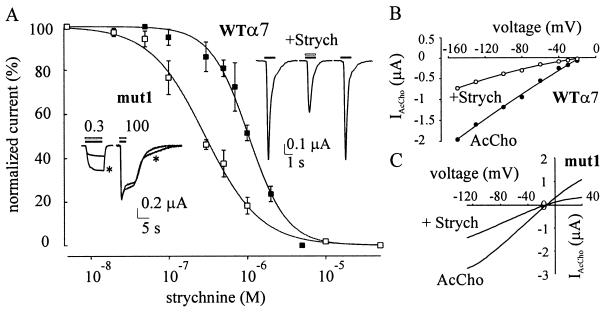
Figure 1.
Effects of strychnine on AcCho-induced currents. (A) Strychnine dose/AcCho current response relations in oocytes expressing WTα7 (■) or mut1 receptors (□). Peak currents, evoked by AcCho (100 μM in ■; 0.3 μM in □) coapplied with strychnine and normalized to the response to AcCho alone. Data represent mean ± SEM (6/3 in ■; 7/2 in □). Data without bars, mean of two oocytes. (Inset, Left) Sample superimposed IAcCho elicited by AcCho at indicated concentrations (in μM), alone (∗), or together with strychnine (0.3 μM) in an oocyte expressing mut1 receptor. (Inset, Right) Sample IAcCho elicited by 100 μM alone (horizontal solid bar) or together with strychnine (1 μM; middle trace) in an oocyte expressing WTα7. (B) Current-voltage relation from one oocyte held at various potentials: (●) 100 μM AcCho and (○) 100 μM AcCho coapplied with 1 μM strychnine (representative of four oocytes, one donor). AcCho was applied at 3-min intervals. Solid lines represent second-order polynomial fits to the data. (C) Current-voltage relations in one oocyte exposed to AcCho alone or AcCho plus strychnine (0.3 μM). Representative of five oocytes, two donors.
It has been reported that the inhibitory action of brief applications of strychnine in oocytes expressing WTα7 nAcChoR increases when the oocytes are pretreated with strychnine (4). Therefore, experiments were carried out exposing the oocytes to strychnine (1 μM) alone, before applying strychnine together with AcCho. Pretreating the oocytes for 3–4 min slightly increased the strychnine-induced IAcCho inhibition, which remained the same when the pretreatment was prolonged to 8 min (not shown).
To investigate whether the inhibitory effects of strychnine on WTα7 receptors were voltage dependent, as recently reported for neuronal heteromeric nAcChoRs (1), current-voltage relationships were examined. These showed the usual rectification at positive potentials in standard Ringer’s solution (11) and were not modified substantially by strychnine (1 μM; Fig. 1B).
Strychnine Blocks mut1 Receptors.
To investigate further the action of strychnine, we also studied its effect on oocytes injected with mut1 subunit cDNA. With AcCho applied at the concentration of 0.3 μM (≈EC50, ref. 11), IAcCho peaked to −895 ± 181 nA (range: −40 nA to 5.3 μA; 31/5) and decayed slowly (T0.1 = 2.8 s in three cells and T0.1 > 4 s in 10 cells). As for WTα7 receptors, strychnine (0.1 nM-1 mM) alone, again did not elicit any obvious current in oocytes expressing mut1, and when coapplied with AcCho, it reduced the AcCho-current amplitude. The IC50 and nH values were 0.29 μM and 1.23, respectively (7/2; Fig. 1A). Furthermore, in four oocytes tested, the peak amplitude of IAcCho (0.3 μM AcCho) was reduced by strychnine (0.3 μM) to 37.4 ± 5% (4/1), and when the AcCho concentration was increased to 100 μM, IAcCho inhibition was completely suppressed (see Fig. 1A, Inset), indicating that strychnine acts as a competitive antagonist on both mut1 and WTα7 receptors. Furthermore, the inhibitory effect of strychnine was voltage-independent because the IAcCho-voltage relationship was rather linear in the presence of strychnine (8/2; e.g., Fig. 1C), even when its concentration was raised to as high as 200 μM (not shown).
Strychnine Activates mut2 Receptors.
To investigate whether mutations of the AcCho binding site would alter the antagonistic action of strychnine, we injected oocytes with a C189–190Sα7 mutant cDNA. Even 5 days after injection, these oocytes (23/3) were unresponsive to AcCho (20 μM-1 mM), either because of a lack of receptor expression and assembly in the membrane or the expression of receptors incapable of being activated by AcCho. We favor the last hypothesis because a 2.8-fold increase in α-BuTx binding, versus the control (15 noninjected oocytes), was observed in 15 oocytes (two donors) injected with the C189–190Sα7 mutant cDNA and assayed for membrane binding (21). Other experiments, performed in oocytes injected with the mut2 cDNA, showed that AcCho applied to these oocytes elicited a dose-dependent inward current (Fig. 2). With 35 μM the AcCho-current amplitude was −617 ± 83 nA (30/4; range 60 to 1, 355 nA), and the current desensitized slowly with a T0.1 = 2.5 ± 1.8 s in seven of nine examined cells and T0.1 > 4 s in the two remaining oocytes, similar to mut1 (Fig. 2, Inset). The AcCho dose-response relationship (Fig. 2) gave EC50 and nH values of 38 μM and 1.7, respectively (12/3), indicating a 130-fold decrease in AcCho affinity for mut2 compared with that of L247Tα7 receptors, but only a 3-fold increase compared with that of wild-type receptors (11). Furthermore, as in mut1-expressing oocytes, the I–V relationship was fairly linear (5/2; Fig. 3A) with AcCho concentrations in the range of 30 to 500 μM. In 15 oocytes injected with mut2 and assayed for toxin binding (the same two donors as used above) we again observed a 2.8-fold increase in α-BuTx binding (not shown), indicating that the C189–190Sα7 mutant expresses AcCho receptors that bind α-BuTx but are not gated by AcCho, or if they are gated their channel conductance is much smaller than the wild-type or mut 1 and mut2 receptors.
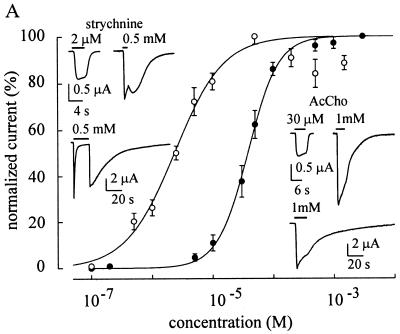
Figure 2.
Effects of AcCho and strychnine on oocytes expressing mut2 receptors. AcCho dose/IAcCho relationship (●) and strychnine dose/Istrych relationship (○) best-fitted to a single Hill equation. Peak IAcCho and Istrych were normalized to that evoked by 3 mM AcCho (IAcCho mean amplitude: −2.8 μA) or by 50 μM strychnine (Istrych mean amplitude: −1.5 μA), respectively. Each point is the mean ± SEM (●, 12/3; ○, 24/8). Note expected decrease of Istrych values at high strychnine concentrations, probably because of a channel block mechanism. (Inset, Right) Sample records of IAcCho in two oocytes: (Upper) oocyte held at −60 mV; (Lower) another oocyte held at −100 mV. (Inset, Left) Sample records of Istrych in two other oocytes, held as at right. Note absence of shoulder in the IAcCho elicited by 1 mM AcCho at right and its presence in Istrych (at −60 mV), and wash kick with long application of 0.5 mM strychnine at −100 mV.
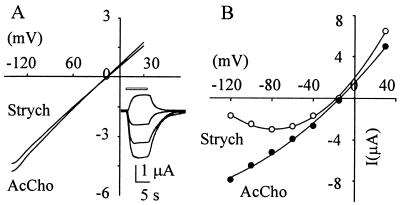
Figure 3.
I–V relationships in oocytes expressing mut2 receptors. (A) Voltage ramps (−130 to 30 mV membrane potential; 1.8-s duration) in the presence of either AcCho (35 μM) or strychnine (5 μM) in the same oocyte. (Inset) Sample records of strychnine currents from another oocyte tested with voltage steps (from bottom to top: −100 mV, −60 mV, −40 mV, +30 mV, inward current, downward). (B) I–V relationships performed with voltage steps showing linearity for IAcCho and rectification at hyperpolarized potential for Istrych. AcCho concentration, 1 mM; strychnine, 500 μM.
In sharp contrast to oocytes expressing WTα7 or mut1, the oocytes expressing mut2 receptors responded to strychnine with a dose-dependent inward current (strychnine-activated current, Istrych; e.g., Fig. 2) which was “nicotinic” in nature, because it was fully blocked by α-BuTx (1 μM; 6/2; not shown). The dose-response curve gave EC50 and nH values of 2.3 μM and 1 (24/8), respectively, indicating that the affinity of strychnine for mut2 was higher than that of AcCho (Fig. 2). With 2.5 μM strychnine the peak Istrych was −819 ± 260 nA (10/4; range −256 to −2, 235 nA) and decayed with T0.1 = 1.4 ± 0.1 s in four cells and T0.1 >4 s in six other cells. Interestingly, using high concentrations (>100 μM), Istrych showed a large “wash kick” (see Fig. 2, Inset). With strychnine concentrations near the EC50, the Istrych-V relationship was linear, like the IAcCho-V relationship, but this linearity was not maintained with concentrations of strychnine over 100 μM (Fig. 3). This result together with the wash kick suggests an open-channel blockage mechanism (22).
To investigate whether strychnine alters the affinity of mut2 receptors for AcCho, dose-response relationships were determined in the presence or absence of strychnine. In five oocytes, strychnine (3 μM ≈ EC50) increased the EC50 value for AcCho by 160%, suggesting a competitive strychnine agonism for a common binding site on the receptor. In contrast, in seven oocytes, strychnine dose-response relationships obtained in the presence of AcCho (35 μM ≈ EC50) gave a mean EC50 value the 60% smaller than the control. These findings suggest a different action of strychnine on the AcCho binding sites, compared with AcCho.
Channels Gated by AcCho and Strychnine Action on mut2 Receptors.
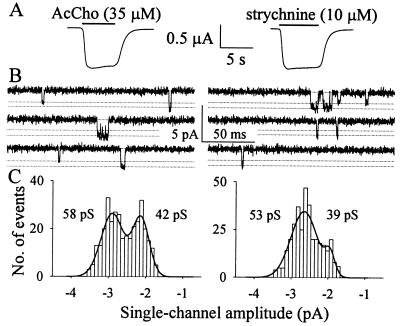
AcCho evoked single-channel activity in three of 11 outside-out patches obtained from mut2 cDNA-injected oocytes (3/2) that responded well to both AcCho and strychnine in voltage-clamp experiments (Fig. 4A). With 35 μM AcCho in the patch pipette, there were two single-channel conductances, 59 pS and 45 pS (Fig. 4 B and C), similar to values previously reported for oocytes expressing mut1 (20). No transitions from the higher to the lower conductance were observed, suggesting that the conductances correspond to the opening of two different channel populations rather than of substates of the same channel population. The larger channel openings, estimated as about 60% of the total, exhibited a mean open time of 3.2 ± 0.4 ms (n = 1,664), and a mean burst duration of 4.7 ± 0.6 ms (n = 1,240), indicating that flickering activity was practically absent under our conditions. On the same outside-out patches, the channels activated by strychnine (10 μM) were similar to those activated by AcCho (Fig. 4). Strychnine-gated unitary currents again showed two conductance levels, 56 pS and 42 pS, with no transitions from higher to lower amplitude. The higher conductance level, representing about 80% of the channels activated by strychnine, exhibited similar kinetics to AcCho channels with no channel flickering and a mean open time and burst duration of 3.9 ± 0.3 ms (n = 3,272) and 5.2 ± 0.5 ms (2,616), respectively.
Figure 4.
Properties of mut2 channels activated by AcCho (Left) or strychnine (Right), in one oocyte injected with mut2 cDNA. (A) Macroscopic voltage-clamped currents. Holding potential, −100 mV. (B) Single-channel currents elicited by AcCho and strychnine in one outside-out patch. Dashed lines indicate amplitude levels of elementary inward currents (downward deflections). Holding potential, −50 mV. (C) Distributions of single-channel amplitudes, detected from recordings shown in B and best-fitted by the sum (thick line) of two Gaussian functions. Unitary conductances as indicated.
Properties of Hybrid AcChoRs Composed of Both mut1 and mut2 Subunits.
Because mut1 and mut2 receptors have different properties and affinities for AcCho and strychnine, we were interested in investigating the characteristics of receptors composed of different subunits. For that purpose, the mut1 and mut2 subunits were coinjected and the properties of the resulting receptors were examined. Oocytes injected with a mixture of mut1 and mut2 subunit cDNAs (injection ratio 1:1) showed inward currents activated by both AcCho and strychnine (Fig. 5 and Table 1) and inhibited by α-BuTx (1 μM; not shown). The time courses of the IAcCho and Istrych evoked in these oocytes were similar to those elicited in oocytes injected with mut1 or mut2 alone (see for instance Fig. 5 vs. Figs. 1 and 2), and the same applied to I–V relations of IAcCho (AcCho, 0.3 μM to 1 mM) and Istrych (strychnine, 2 μM to 500 μM) (not shown).
Figure 5.
Membrane currents from oocytes injected with a mixture of cDNA encoding mut1 and mut2 subunits with ratio of 1:1. AcCho dose/IAcCho relationship (●) and strychnine dose/Istrych relationship (○) both best-fitted to a single Hill equation from the same oocytes. For AcCho the EC50 and nH values determined from the fit were 0.77 μM and 1.9, respectively. The AcCho currents were normalized to that evoked by 10 μM AcCho (IAcCho mean amplitude: −2.4 μA). For the same oocytes strychnine EC50 and nH values were 1.3 μM and 1.4, respectively. Strychnine currents were normalized to that evoked by 50 μM strychnine (Istrych mean amplitude: −0.92 μA). Each point represents the mean ± SEM (10/2). Dashed line represents the theoretical curve for the expression of mut1 and mut2 receptors and lack of hybrids (values of EC50 and nH for pure mut1 and mut2 receptors, as in Table 1). (Insets) Sample IAcCho (Left) and Istrych (Right) at indicated concentrations.
Table 1.
Current responses of oocytes injected with single subunit cDNAs or a mixture of mut1 and mut2 subunit cDNAs
| Subunit cDNAs | AcCho
|
Strychnine
|
||||||
|---|---|---|---|---|---|---|---|---|
| EC50 (μM) | nH |
I(nA)
|
EC50 (μM) | nH |
I(nA)
|
|||
| 0.3 μM | 1 mM | 1 μM | 0.5 mM | |||||
| mut1 | 0.27 | 1.5* | 884 ± 2635 | 1,895 ± 3381 | — | 0 | 0 | |
| mut2 | 38 | 1.7 | 0 | 2,610 ± 2533 | 2.3 | 1.0 | 568 ± 1004 | 1,544 ± 1382 |
| (mut1/mut2) (1:1) | 0.77 | 1.9 | 225 ± 775 | 1,857 ± 3001,3 | 1.3 | 1.4 | 393 ± 934 | 1,110 ± 2562 |
Peak current amplitudes (mean ± SEM) from oocytes injected with the indicated cDNAs. Data from 5–24 oocytes, 3–8 donors). ∗, ref. 11. Student’s t test, P > 0.25 for data sets with numbers 1 and 2; P < 0.05 for numbers 3, 4, and 5. Note lack of EC50 value for strychnine on mut1 because of its antagonistic action.
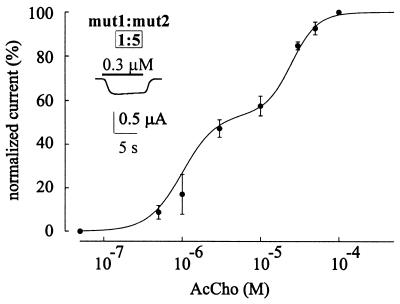
Coexpression of mut1 and mut2 subunits should result in dose-response relationships having at least three components resulting from activation of mut1, mut2, and hybrid receptors, respectively. However, in 10 oocytes (two donors) the AcCho dose-IAcCho response relationship was best-fitted to a single Hill equation whereas the theoretical curve predicted by the expression of only pure mut1 and mut2 receptors did not fit the experimental data well (Fig. 5), suggesting that the expression of hybrid receptors is favored, and that pure mut1 and mut2 receptors are not numerous (see Fig. 6). In the same oocytes the strychnine dose-Istrych relationship again was best-fitted to a single Hill equation (Fig. 5). On the assumption that by changing the cDNAs injection ratio the probability of subunit combinations is correspondingly changed in agreement with a binomial distribution probability (Fig. 6) the expression of hybrid populations is further supported by the dose-response relationship for AcCho in oocytes in which the cDNA injection mut1/mut2 ratio was changed from (1:1) to (1:5). This relationship was best-fitted to an equation composed by the sum of two Hill equations (Fig. 7), with EC50, nH, and component weight values resembling those of both the mut2 and hybrid receptors (see Fig. 7 vs. Table 1 and Fig. 6).
Figure 6.
Diagram illustrating the pentameric symmetrical model and predicted hybrid nAcChoR diversity. Numbers represent fractions of the eight possible combinations determined by the binomial equation assuming equal efficiency in the expression and assembly of mut1 or mut2 subunits. (Upper Numbers) cDNA injection ratio 1:1; (Lower Numbers) ratio 1:5. The binomial equation used to calculate the above fractions was: prqn-r × n!/[r!(n − r)!], where p and q are the incorporation probabilities of mut1 or mut2 subunits, and n is the number of subunits in the receptor complex (five) taken r subunits at a time. Note higher probability (about 60%) for combinations two vs. three subunits in the ratio 1:1.
Figure 7.
AcCho dose/IAcCho response relationship best-fitted to a sum of two Hill equations in oocytes injected with a mixture of cDNA encoding mut1 and mut2 subunits with ratio of 1:5. The EC50, nH, and weight values determined from the fit were: 1 μM, 2, and 55%, respectively (first component), and 25 μM, 2.5 and 45%, respectively (second component). The peak AcCho currents were normalized to that evoked by 100 μM AcCho (IAcCho mean amplitude: −1.4 μA). Each point represents the mean ± SEM (6/2). A single Hill equation fit was statistically less significant (F-distribution test). (Inset) Sample IAcCho.
Discussion
The α7 neuronal nAcChoR subunit is expressed mainly in the central nervous system (23). In Xenopus oocytes, the α7 subunit forms α-BuTx-sensitive homomeric nAcChoRs that are noncompetitively inhibited by a variety of antagonists, such as curare, hexamethonium, dihydro-β-erythroioline (DHβE), 5-hydroxytryptamine, and Zn2+. These are converted into agonists by the L247T (mut1) point mutation in the leucine ring of the M2 channel domain (10, 11, 19).
Strychnine, the potent glycine receptor antagonist, recently has been discovered to also be a potent nicotinic antagonist at neuronal nicotinic heteromeric and homomeric receptors (1, 2, 4–7). We tested strychnine on Xenopus oocytes expressing either WTα7 or mut1 nAcChoRs and found conclusive evidence that strychnine is a competitive blocker not only of WTα7 nAcChoRs, in agreement with previous reports (4–7), but also of mut1 receptors. Thus, our findings place strychnine among a class different from that at the nicotinic α7 receptor antagonists that become agonists after the L247T mutation (10). We report also that strychnine does not change the desensitization or voltage-dependence of WTα7 or mut1 receptors, but acts on them as a “pure” competitive blocker. Nonetheless, in some heteromeric neuronal nAcChoRs, the strychnine block is noncompetitive and voltage dependent (1). Thus, our findings taken together with previous data (1, 2, 4–7) suggest avenues for the design of novel therapeutic agents for neurodegenerative diseases such as Alzheimer’s and Parkinson’s, at which the α7 subunit appears to play a role in both disease genesis and progression (15).
A large body of evidence has identified Cys-192 and Cys-193 as being in, or near, the AcCho-binding site in the N terminal of the Torpedo α1 subunit. These residues correspond to Cys-189 and Cys-190 in the analogous region of the chick α7 subunit (13–15, 24, 25). A full understanding of the action of strychnine on α7 nAcChoR requires the identification of the strychnine binding sites. Toward that goal we mutated the Cys-189 and Cys-190 residues and found that AcCho failed to elicit a response, presumably because the C189–190Sα7 mutant receptors are nonfunctional. In contrast, we found that AcCho induced large currents in oocytes expressing mut2 receptors. Moreover, these receptors are also potently activated by strychnine, which therefore is converted from an antagonist into an agonist. Interestingly, we also found that although AcCho does not reduce the affinity for strychnine and behaves as a noncompetitive agonist, strychnine shifts the dose-response curve of AcCho to the right, behaving as a competitive agonist of AcCho at mut2. We conclude that strychnine shares part of its binding site with AcCho, but has a more complex binding area than AcCho at α7 receptors. Interestingly, the WTα7 subunits mutated in the AcCho binding site at Cys-189 and Cys-190 are expressed in the cell membrane but seem to be not assembled into functional receptors. Thus, our results suggest that the Cys-189–190 site may be involved in binding and gating of WTα7 receptors, and that the wild-type and mut2 receptors have at least another AcCho binding site that is sufficient for gating L247T and mut2. Furthermore, that mutations in residues within the binding area make it possible for strychnine to gate the receptor channels confirms that complex interactions are taking place between the leucine ring and the transmitter binding area, as previously revealed by the agonistic action of several drugs and ions on mut1 (10, 11, 19).
It is commonly thought that muscle nAcChoR consists of a ring of five similar subunits with an AcCho binding site in each of the twin α-subunits (26, 27) and that neuronal homomeric and heteromeric nAcChoRs also have a pentameric architecture (16, 28, 29). Specifically, the heteromeric neuronal receptor complex presumably consists of two AcCho-binding α-subunits and three β-subunits. It is less clear how many AcCho-binding subunits determine channel activation and receptor functional properties. For example, in the particular case of the homomeric α7 receptor, we do not know whether there is a hierarchy within the α-subunits or whether they are five equivalent and independent subunits. In this paper we used the different properties of mut2 vs. mut1 as a tool to address that question. We found that injection of equal amounts of mut1 and mut2 led to the expression of nicotinic receptor hybrids in ratios agreeing with a binomial hypothesis of subunit coassembly, supporting the view that, under our experimental conditions, the receptor populations share a pseudosymmetrical, pentameric structure that favors the heteromeric rather than the homomeric receptors. Moreover, because hybrid receptors are activated by both AcCho and strychnine, it is likely that one or two mut2 subunits might be sufficient to shift strychnine from an antagonist at mut1 into an agonist at hybrid receptors, without substantially affecting the AcCho affinity as determined for the pure mut1. Given that with injection ratio (1:1) the more probable subunit combination within a pentameric structure is two vs. three subunits, we speculate that two α-subunits mediate the functional properties of the homomeric receptors, with the remaining three behaving as structural subunits.
Acknowledgments
We thank Drs. Marc Ballivet and Jesus Garcia-Colunga for critical reading of the manuscript and Dr. Anna Maria Mileo for help in mutating the α7 subunit. This work was supported in part by Ministero Università e Ricerca Scientifica (to F.E.) and by a grant from Consejo Nacional de Ciencia y Technología (Mexico) (to R.M.).
Abbreviations
- AcCho
acetylcholine
- nAcChoR
nicotinic AcCho receptor
- IAcCho
AcCho-activated current
- Istrych
strychnine-activated current
- WTα7
wild-type α7 nAcChoR
- α-BuTx
α-bungarotoxin
References
- 1.Garcia-Colunga J, Miledi R. Proc Natl Acad Sci USA. 1999;96:4113–4118. doi: 10.1073/pnas.96.7.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsubayashi H, Alkondon M, Pereira E F, Swanson K L, Albuquerque E X. J Pharmacol Exp Ther. 1998;284:904–913. [PubMed] [Google Scholar]
- 3.Anand R, Peng X, Lindstrom J. FEBS Lett. 1993;327:241–246. doi: 10.1016/0014-5793(93)80177-v. [DOI] [PubMed] [Google Scholar]
- 4.Anand R, Peng X, Ballesta J, Lindstrom J. Mol Pharmacol. 1993;44:1046–1050. [PubMed] [Google Scholar]
- 5.Gerzanich V, Anand R, Lindstrom J. Mol Pharmacol. 1994;45:212–220. [PubMed] [Google Scholar]
- 6.Peng X, Katz M, Gerzanich V, Anand R, Lindstrom J. Mol Pharmacol. 1994;45:546–554. [PubMed] [Google Scholar]
- 7.Rothlin C V, Katz E, Verbitsky M, Elgoyhen A B. Mol Pharmacol. 1999;55:248–254. doi: 10.1124/mol.55.2.248. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Vijayaraghavan S, Berg D K. Neuron. 1994;12:167–177. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 9.Revah F, Bertrand D, Galzi J L, Devillers-Thiery A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux J P. Nature (London) 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand D, Devillers-Thiery A, Revah F, Galzi J L, Hussy N, Mulle C, Bertrand S, Ballivet M, Changeux J P. Proc Natl Acad Sci USA. 1992;89:1261–1265. doi: 10.1073/pnas.89.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palma E, Mileo A M, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1996;93:11231–11235. doi: 10.1073/pnas.93.20.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maggi L, Palma E, Miledi R, Eusebi F. Mol Psychiatry. 1998;3:350–355. doi: 10.1038/sj.mp.4000392. [DOI] [PubMed] [Google Scholar]
- 13.Karlin A. Curr Biol. 1993;3:299–309. doi: 10.1016/0959-4388(93)90121-e. [DOI] [PubMed] [Google Scholar]
- 14.Galzi J-L, Changeux J-P. Curr Biol. 1994;4:554–565. [Google Scholar]
- 15.Lindstrom J. In: Ion Channels. Narahashi T, editor. Chicago: Plenum; 1996. pp. 377–450. [Google Scholar]
- 16.Palma E, Bertrand S, Binzoni T, Bertrand D. J Physiol (London) 1996;491:151–161. doi: 10.1113/jphysiol.1996.sp021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertrand S, Devillers-Thiery A, Palma E, Buisson B, Edelstein S J, Corringer P J, Changeux J P, Bertrand D. NeuroReport. 1997;8:3591–3596. doi: 10.1097/00001756-199711100-00034. [DOI] [PubMed] [Google Scholar]
- 18.Miledi R, Parker I, Sumikawa K. Proc R Soc London Ser B. 1983;218:481–484. doi: 10.1098/rspb.1983.0053. [DOI] [PubMed] [Google Scholar]
- 19.Palma E, Maggi L, Miledi R, Eusebi F. Proc Natl Acad Sci USA. 1998;95:10246–10250. doi: 10.1073/pnas.95.17.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palma E, Maggi L, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1997;94:9915–9919. doi: 10.1073/pnas.94.18.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palma E, Maggi L, Barabino B, Eusebi F, Ballivet M. J Biol Chem. 1999;274:18335–18340. doi: 10.1074/jbc.274.26.18335. [DOI] [PubMed] [Google Scholar]
- 22.Colquhoun D, Ogden D C. J Physiol (London) 1988;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Role L W, Berg D K. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 24.Karlin A, Akabas M H. Neuron. 1995;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 25.Galzi J-L, Bertrand D, Devillers-Thiery A, Revah F, Bertrand S, Changeux J P. FEBS Lett. 1991;294:198–202. doi: 10.1016/0014-5793(91)80668-s. [DOI] [PubMed] [Google Scholar]
- 26.Bertazzon A, Conti-Tronconi B M, Raftery M A. Proc Natl Acad Sci USA. 1992;89:9632–9636. doi: 10.1073/pnas.89.20.9632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lal R, Yu L. Proc Natl Acad Sci USA. 1993;90:7280–7284. doi: 10.1073/pnas.90.15.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anand R, Conroy W G, Schoepfer R, Whiting P, Lindstrom J. J Biol Chem. 1991;17:11192–11198. [PubMed] [Google Scholar]
- 29.Cooper E, Couturier S, Ballivet M. Nature (London) 1991;350:235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]