Abstract
Hibernation torpor provides an excellent natural model of tolerance to profound reductions in blood flow to the brain and other organs. Here, we report that during torpor of 13-lined ground squirrels, massive SUMOylation occurs in the brain, liver, and kidney. The level of small ubiquitin-related modifier (SUMO) conjugation coincides with the expression level of Ubc9, the SUMO specific E2-conjugating enzyme. Hypothermia alone also increased SUMO conjugation, but not as markedly as hibernation torpor. Increased SUMO conjugation (induced by Ubc9 overexpression, ischemic preconditioning (PC)±hypothermia) was necessary and sufficient for tolerance of SHSY5Y neuroblastoma cells to oxygen/glucose deprivation (OGD) (‘in vitro ischemia’); decreased SUMO conjugation (induced by a dominant-negative Ubc9) severely reduced tolerance to OGD in these cells. These data indicate that post-translational modification of proteins by SUMOylation is a prominent feature of hibernation torpor and is critical for cytoprotection by ischemic PC± hypothermia in SHSY5Y cells subjected to OGD.
Keywords: hypothermia, oxygen–glucose deprivation, tolerance
Introduction
Stroke is a devastating disorder that affects approximately 700,000 people every year and leads to a death approximately every 3 mins in the United States according to American Heart Association 2006 statistics. To counter these grim statistics, it is crucial to find new strategies for preventing and treating stroke.
Identification of the molecular mechanisms that regulate states of tolerance to ischemia can guide development of new therapeutic strategies for human vascular diseases including stroke (Dirnagl et al, 2003). Mammalian hibernation serves as a natural model of tolerance to extreme reduction of blood flow and the capacity to deliver oxygen to tissues (Frerichs and Hallenbeck, 1998; Frerichs et al, 1994). During torpor, hibernating animals lower their energy consumption, blood flow, and body temperature to otherwise lethal levels, but because of their special adaptive changes, they suffer no CNS damage or cellular loss (reviewed in Carey et al, 2003; Storey, 2003). The molecular mechanisms that regulate these adaptive changes are potential targets for drug discovery.
The limited energy availability in hibernation torpor favors post-translational modification of proteins more than energy consuming de novo protein synthesis as a regulatory mechanism for tolerance. The covalent attachment to proteins of the small ubiquitin-related modifier (SUMO) has received much attention since its discovery (Mahajan et al, 1997; Matunis et al, 1996) because of its intriguing and essential functions. The SUMOylation cycle involves at least four enzymes in a multistep process. The E1-activating enzyme, ubi-quitin-associated2/activation of Smt3p1, initiates the first step. Then the SUMO specific E2-conjugating enzyme, Ubc9, receives the activated SUMO and transfers it to conjugate with a substrate protein, sometimes with help of an E3-ligase. A group of isopeptidases (SENPs) that deconjugate SUMO-ylated proteins complete the cycle (reviewed in Hay, 2005; Muller et al, 2001). Small ubiquitin-related modifier affects proteins involved in gene expression, chromatin structure, signal transduction, and maintenance of the genome (reviewed in Hay, 2005). Transcription factors are the major targets for SUMO conjugation and SUMOylation of these proteins mainly produces negative effects on gene expression (Girdwood et al, 2004). This prompted us to determine whether SUMO conjugation status changes during the metabolic rate suppression of hibernation torpor. There are three SUMO paralogues in mammals. Two, SUMO-2 and SUMO-3, are 96% identical in sequence and are difficult to distinguish. In contrast, SUMO-1 is only 45% identical with the other two SUMO paralogues and has distinct immunoreactivity (Lapenta et al, 1997).
We find that massive protein SUMOylation occurs in 13-lined ground squirrels (Spermophilus tridece-milineatus) during their torpor phase of hibernation in multiple organs and that a portion of this SUMO conjugation is induced by hypothermia itself. We examined whether changes in SUMO conjugation levels in SHSY5Y cells affect their level of tolerance to oxygen/glucose deprivation (OGD), an in vitro model of ischemia. Increased protein SUMOylation (induced by Ubc9 overexpression or ischemic pre-conditioning (PC) with or without hypothermia) confers equivalent levels of tolerance and decreased protein SUMOylation (induced by a dominant–negative (DN) Ubc9) severely decreases resistance to OGD in these cells.
Materials and methods
Animal Preparation
Thirteen-lined ground squirrels, S. tridecemlineatus, were captured by USDA-licensed trappers (TLS Research, Bartlett, IL, USA). Experiments were approved by the NINDS Animal Care and Use Committee. Both male and female squirrels were used equally for this study, and all animals were between one and three years of age, but because the animals were caught from the wild there is no way to know the exact age of the animals. Ground squirrels were housed individually at 21°C under a 12 h light:12 h dark cycle, and were fed standard rodent diet and water ad libitum. To facilitate hibernation torpor, the ground squirrels were transferred to an environmental chamber maintained at 4°C to 5°C and 60% humidity and placed separately in cages containing wood shavings (Frerichs et al, 1994). They were kept in darkness, except for a photographic red safe light (3 to 5 lux). Body temperature (Tb) was measured with an Implantable Programmable Temperature Transponder IPTT-200 (Bio Medic Data Systems). To implant the temperature transmitters, the ground squirrels were first anaesthetized with 5% isofluorane and then the transmitter was injected subcutaneously into the middle of the back using a sterile disposable syringe. Animals in seven different phases of the hibernation bout (cycle) were differentiated by body temperature (Tb), time, and respiratory rate. ART= active animals at 21°C (Tb = 34°C to 37°C); ACR= active for 4 to 5 days in the environmental chamber at 4°C to 5°C (Tb = 34°C to 37°C). ACR represents animals that are capable of entering torpor, but have yet to do so. EN= entrance phase of hibernation (Tb = 31°C to 12°C) after > 14 h in the active state since full arousal from a torpor phase of at least 5 days. Torpor phase animals (TOR) were eligible after they had been in the torpor phase for more than 5 days, aroused and then re-entered the torpor phase. ET = early torpor phase (1 day) (Tb = 5°C to 8°C); LT = later torpor phase (> 5 days) (Tb = 5°C to 8°C); AR= arousing from torpor spontaneously with a respiratory rate > 60/min and a persistent low body temperature (Tb = 9°C to 12°C). 7hIA = interbout aroused from torpor animals are controls that have previously been in the torpor phase of the hibernation bout, but have since returned to normal metabolic conditions in the active state inside the environmental chamber for 7 h (Tb = 34°C to 37°C). The 7 h extends from the point that the ground squirrel body temperature has returned to 37°C.
Brain, heart, skeletal muscle, kidney, liver, thymus, and spleen were removed quickly, washed with chilled 0.15 mol/L NaCl, frozen instantly in 2-methylbutane (−50°C), cooled on dry ice, and then stored at −70°C.
Tissues from both male and female summer active ground squirrels were collected in the first week of July. Body weights ranged from 190 g up to 300 g.
We lowered body temperature in thirteen-lined ground squirrels that were not hibernating to control for the effects of hypothermia alone. Ground squirrels were anesthetized by intraperitoneal injection of 50 mg/kg pentobarbital sodium. When these anesthetized animals were placed in the cold chamber, their body temperature decreased gradually to levels that corresponded to that of animals entering hibernation. These animals were euthanized by decapitation when their body temperatures reached 24°C.
Western Blot Analysis
Frozen brain (whole or neuroanatomic regions) or other organs were crushed on dry ice to make powder, added to 2% SDS, 60 mmol/L Tris-HCl (pH 6.8), 50 mmol/L EDTA, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerol-phosphate, 1 mmol/L PMSF and 1 µg/mL Leupeptin, and homogenized on ice. The homogenates were sonicated for 10 to 15 secs, boiled for 5 mins at 95°C and centrifuged at 15,000g for 10 mins at 4°C. After the protein concentration was measured, the supernatant was boiled again with 5% β-mercaptoethanol and 2% glycerol, and subjected (30 µg/lane) to SDS-PAGE (4% to 20%). Western blot analyses were performed using the following antibodies: rabbit polyclonal anti-SUMO-1 antibodies raised against the processed form of human SUMO-1 (1:1000) (Azuma et al, 2003), rabbit polyclonal anti-SUMO-2/3 antibodies (1:1000) provided by Saito (Saitoh and Hinchey, 2000), rabbit polyclonal anti-human SENP-1 antibodies (1:1000) provided by M Dasso, mouse monoclonal anti-Ubc9 (1:500) purchased from BD Biosciences, and mouse monoclonal antiactin (β and total) antibodies (1:10,000, and 1:5000, respectively) purchased from Sigma Chemicals. Intensities of bands were analyzed by the Macintosh densitometry program ImageJ (NIH). For SUMO conjugate band analysis, the higher molecular weight area (100 to 250 kDa) in each lane was cropped and analyzed.
Immunohistochemistry
Animal brains were excised and fixed for 24 h in 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) (PB) at 4°C and stored in 20% sucrose in 0.1 mol/L PB. Serial sections (20 µm thickness) were made on a cryostat, and were incubated overnight at 4°C with either anti-SUMO-1 or anti-SUMO-2/3 (both 1:10,000 dilution) in PBS with 0.3% Triton X-100 (PBSTx). After 15 mins wash in PBSTx, the sections were incubated with biotinylated anti-rabbit IgG for 1 h at RT followed by an hour incubation with avidin-biotin peroxidase complex solution. The immunoreaction products were visualized with a diaminobenzidine substrate. Multiple SUMOylated proteins are detected by these antibodies. Some of the sections that were immunostained with SUMO-1 or SUMO-2/3 antibodies were counterstained with hematoxylin.
Confocal Microscopy
Brains were excised and immediately frozen on dry ice. Brain cryosections were incubated with 2% BSA in PBS for 1 h at room temperature, then overnight at 4°C with anti-SUMO-1 (1:1000) or anti-promyelocytic leukemia (PML) antibodies (1:200) (rabbit polyclonal anti-PML antibodies raised against C-terminus of PML isoform IV; Chemicon International). These anti-PML antibodies detect several isoforms of PML (three major bands corresponding to various isoforms were detected on Western blot). The sections were washed three times with 2% BSA in PBS and incubated with Alexa Fluor 594- or 488-conjugated anti-rabbit IgG (Molecular probes) for 2 h at RT. After three 10 mins washes with PBS, Hoechst 33,342 (Sigma chemicals) was added at 1 µg/mL in PBS to stain nuclei. Images were captured with a confocal microscope (model LSM 510; Carl Zeiss Micro Imaging) using a 63 × 1.4 NA Apochrome objective. We used a 543 nm HeNe laser for Alexafluor 594-labeled proteins, an Argon laser for Alexafluor 488-labeled proteins and UV (364 nm) laser for Hoechst 33342 images. Projected series of z-sections collected with intervals of 0.5 to 0.75 µm are shown.
Cell Culture, Transfection and Generation of Tet-Inducible Stable Transfectants
The human neuroblastoma cell line SHSY5Y (American Type Culture Collection, Manassas, VA, USA) was cultured in complete Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 100U/mL penicillin, and 100 µg/mL streptomycin in 5% CO2 at 37°C. Transfections of SHSY5Y cells were performed by Nucleofector (Amaxa, Inc.), according to the manufacturer’s instructions. A tetracycline (tet)-regulated expression system (T-Rex system; Invitrogen) was used for generating tet-inducible stable transfectants: we first generated a SHSY5Y cell line stably expressing the tet repressor from pcDNA6/TR plasmid (T-Rex-SHSY5Y) (selected by blasticidine 5 to 50 µg/mL) (parent cells). Genes of interest (Ubc9 in this study) were subcloned into pcDNA4/TO/myc-His (or pcDNA4/TO), introduced into T-Rex-SHSY5Y cell lines, and established as stable transfectants by dual selection using zeocin (100 µg/mL) and blasticidin (5 µg/mL). The original constructs of mouse Ubc9 (WT), mouse DN mutant Ubc9 (C93A L97A) or Xenopus DN mutant (C93S L97S) were kind gifts from Mary Dasso (Azuma et al, 2003; Saitoh et al, 1997).
Oxygen and Glucose Deprivation and Preconditioning (Hillion et al, 2005) of SHSY5Y Cells
SHSY5Y cells were plated at a density of 5 × 105 cells/well in six-well plates precoated with poly-D-lysine (BD Biosciences, Bedford, MA, USA) and grown for 24 h in complete DMEM supplemented with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere. For the induction of OGD, cells were washed twice in DMEM medium without glucose (Invitrogen) supplemented with 2% FBS (OGD medium) and placed in modular incubator chambers (Billups-Rothenberg, Del Mar, CA, USA). The chambers were flushed with a gas mixture of 95% N2/5% CO2 for 30 mins at room temperature at 3 L/mins. After flushing, the chambers were sealed and maintained at 37°C (unless otherwise stated). Oxygne/glucose deprivation was performed for 12 h or for the indicated times (O2 levels 2% to 3%). For PC experiments, cells that were grown for 24 h in complete medium were washed twice with OGD medium and subjected to 6 h of OGD with initial flushing as described above. Control plates that were not subjected to OGD were washed twice with DMEM without glucose and maintained in DMEM (glucose present) supplemented with 2% FBS. Oxygen/glucose deprivation preconditioned cells were then returned to glucose-containing medium and normoxia (‘reperfusion’) for 24 h for development of cellular tolerance. Subsequently, these cells were exposed to severe OGD for 12 h. Control cells were maintained in DMEM supplemented with 2% FBS, but were not exposed to OGD. For stable transfectants, tetracycline (1 µg/mL) was added to the cultures 24 h before OGD.
Assessment of Cell Death: Nuclear Staining with Hoechst 33342 and Propidium Iodide followed by Flourescence-Activated Cell Sorting Analysis
Cells were collected with a papain dissociation system (Worthington Biochemical Corporation, Lakewood, NJ, USA) and 106 cells were labeled with Hoechst 33342 and propidium iodide. The cells were analyzed using a dual-laser FACSVantage SE flow cytometer (Becton Dickinson, Mountain View, CA, USA) as described previously (Hillion et al, 2005). Propidium iodide signals were excited using a 488-nm laser light and their emissions captured using bandpass filters set at 613/20 nm. Hoechst 33342 was excited using a 351-nm UV laser light and its emission captured with a bandpass filter set at 450/20 nm. Cell Quest Acquisition and Analysis software (Becton Dickinson) was used to acquire and quantify the fluorescence signal intensities and to graph the data as bivariate dot-density plots.
Cloning of Ground Squirrel Small Ubiquitin-Related Modifier-1 and Ubc9
Antibodies to human SUMO-1 and clones of mouse Ubc9 were used in these studies. However, we cloned and sequenced SUMO-1 and Ubc9 cDNAs of thirteen-lined ground squirrels and found that while their DNA sequences varied compared with mouse and human sequences, their amino-acid sequences were identical to those of mouse and human (GenBank accession numbers: DQ385870 for ground squirrel SUMO-1, DQ385871 for ground squirrel Ubc9).
Statistics
Exact nonparametric inference via a software package StatXact version 6 (Cytel Software Corporation, Cambridge, MA, USA) was used to account for small data sets. With larger data sets, parametric inference such as twosample t-test was used. To see overall differences among more than two independent groups such as cell groups, the Kruskal–Wallis test (nonparametric version of Analysis of Variance) is used. Trend analyses are implemented via either the Mack-Wolfe test (Mack and Wolfe, 1981) or the Jonckheere-Terpstra (Lehmann, 1975) test if necessary, depending on a pattern of a trend. The Mack–Wolfe test is for umbrella ordering in mean responses among pre-ordered conditions (such as torpor phase) and the Jonckheere–Terpstra test is for either increasingly or decreasingly ordering in mean responses among preordered conditions (such as cell types). Significance for all tests was established at an alpha level of P < 0.05 and data values are given as mean±s.d. of at least three independent experiments.
Results
Massive SUMO-1 and SUMO-2/3 Conjugation Occurs in the Brains of 13-Lined Ground Squirrels During Hibernation Torpor
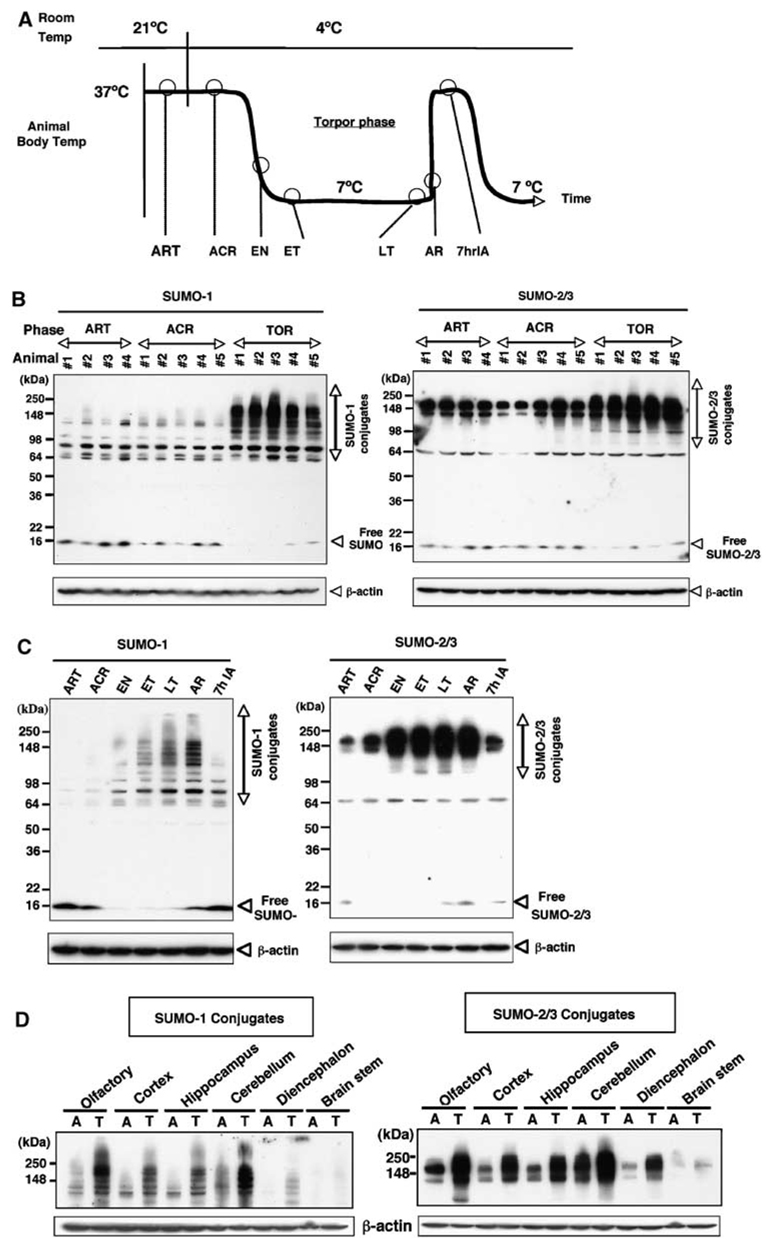
Thirteen-lined ground squirrels, S. tridecemlineatus, were captured during summer, housed individually at 21°C and induced to undergo hibernation during fall-winter (Frerichs et al, 1994). A hibernation bout may last for weeks and consists of entry into, maintenance of, and arousal from torpor. Animals in seven different phases of the hibernation bout were differentiated by body temperature, time, and respiratory rate (Figure 1A). Arousal from torpor is maintained for a few hours to about 1 day before the animal initiates another hibernation bout. We found that extensive SUMOylation (particularly involving formation of high molecular weight, 100 to 250 kDa, SUMO conjugates) by SUMO-1 and SUMO-2/3 occurred in brain tissue samples during torpor in ground squirrels (Figure 1B). In contrast, SUMO conjugation levels were minimal in the brains of winter active animals (ART, ACR (Figure 1B) and in summer animals (Figure 5A and unpublished data). Conjugation of both SUMO-1 and SUMO-2/3 starts to increase on entrance into torpor, progressively increases throughout the torpor phase and returns to baseline levels by 7 h after interbout arousal (Figure 1C). The antibodies for the SUMO paralogues that we used in this study (Azuma et al, 2003) as well as the antibodies available from commercial sources recognized conjugated SUMO better than free SUMO. For this reason, precise quantitation of free SUMO is difficult, but the amount of free SUMO-1 (and perhaps of free SUMO-2/3 as well) appeared to decrease in inverse proportion to the increase in SUMO conjugation (Figure 1C). This relationship between conjugated and free SUMO suggests that the observed SUMO conjugations were at least partially independent of de novo SUMO-1 (or SUMO-2/3) protein synthesis. Small ubiquitinrelated modifier conjugation increased during torpor in all sampled brain regions (Figure 1D). The major SUMO-1 immunoreactive cells in cerebral cortex and cerebellum were neurons (Figure 2A). In active animals, SUMO-1 staining was dispersed throughout the neuronal cell bodies. In contrast, animals in the torpor phase showed nuclear accumulation of SUMO-1 with cortex and in the Purkinje cells of the cerebellum (Figure 2A). We confirmed the nuclear localization of SUMO conjugates in neurons by counterstaining with hematoxylin (Figure 2B) and by confocal microscopy (Figure 2C). In the cortical neurons of active animals, SUMO-1 was found throughout the cell body with some perinuclear emphasis, but in brain sections from torpor phase animals, SUMO-1 was mainly concentrated in the nucleus (Figure 2C). The PML gene encodes a tumor suppressor protein, which was one of the earliest SUMO substrates reported, and its modification by SUMO-1 or SUMO-2/3 is associated with a distinct subnuclear domain, the nuclear body. As shown in Figure 2D, PML nuclear bodies in cortical neurons were increased in both number and size in brain sections from torpid animals.
Figure 1.
Massive SUMO-1 and SUMO-2/3 conjugation occurs during hibernation in the brain of 13-lined ground squirrels. (A) Hibernation bout (cycle) in the 13-lined ground squirrel. ART: active animals at room temperature (Tb=34°C to 37°C); ACR: active animals in cold room (Tb=34°C to 37°C); EN: animals entering torpor (Tb=31°C to 12°C); ET: animals in the early stage of torpor (1 day after entrance into torpor, Tb=5°C to 8°C); LT: animals in the late stage of torpor phase (more than 5 days in torpor, Tb=5°C to 8°C); AR: animals arousing from torpor (Tb=9°C to 12°C); 7hIA: interbout aroused animals that have been in the active state for 7 h (Tb=34°C to 37°C). (B) Immunoblots of SUMO-1 or SUMO-2/3 (free and conjugated forms) in the brain of ART, ACR, and animals in the torpor phase of hibernation bouts (TOR) (n=4 to 5 in each group). (C) Immunoblots of conjugated and free SUMO-1 or SUMO-2/3 in the brain during the phases of the hibernation bout. β-Actin served as a loading control. Each gel is representative of three independent experiments. (D) Immunoblots of protein extracts from different regions of brain (as indicated in the figure) of active (A) and torpor (T) phase animals. β-Actin served as a loading control.
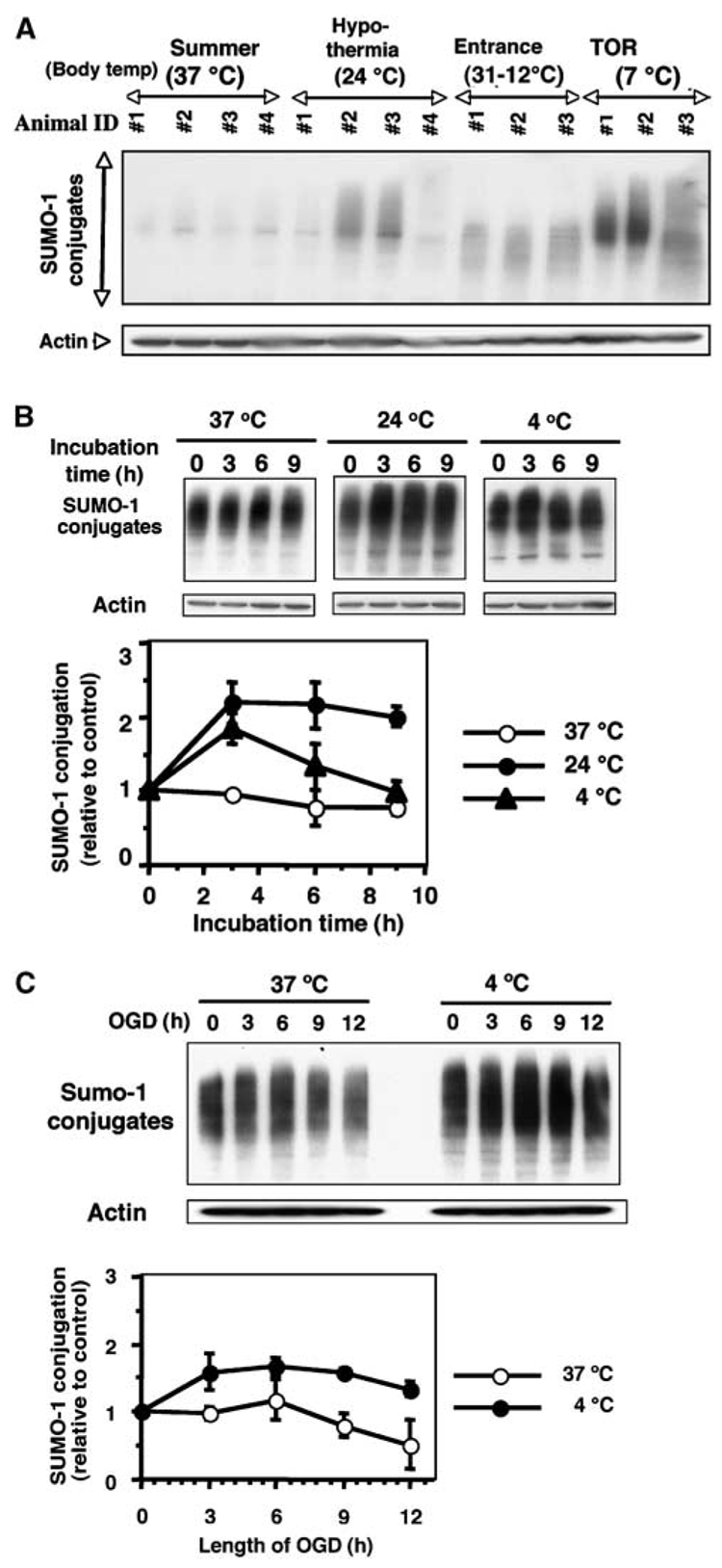
Figure 5.
Effect of temperature (hypothermia) and/or OGD on SUMO-1 conjugation level in SHSY5Y cells. (A) Immunoblots of SUMO-1 conjugated protein in ground squirrel brain under various conditions. Body temperatures are shown in parentheses. Summer animal (37°C), hypothermia (24°C), Entrance (31°C to 12°C) and TOR (7°C). Actin served as a loading control. (B) Immunoblots of SUMO-1 conjugated proteins in total cell lysates of SHSY5Y cells grown at 37°C, 24°C, or 4°C for 0, 3, 6, or 9 h (upper panel). Relative density of SUMO-1 conjugates normalized to actin levels and expressed as the ratio to time zero control cells that were incubated at 37°C (lower panel). (C) Immunoblot of SUMO-1 conjugated protein in total cell lysates of SHSY5Y cells exposed to OGD at 37°C or 4°C for 3, 6, 9, or 12 h (upper panel). Relative density of SUMO-1 conjugates shown as the ratio to time zero cells that were incubated at 37°C (lower panel). Data represent the mean±s.d. of three independent experiments.
Figure 2.
Distribution of SUMO-1 and SUMO-2/3 conjugates in various brain regions and neuronal cells during active and torpor phases of the hibernation bout. (A) Immunohistochemical analysis of the distribution of SUMO-1 in cerebral cortex and cerebellum of ACR and TOR at three different magnifications. Scale bars show 500, 50 and 10 µm (from lower to higher magnification), respectively. Arrows in the photomicrographs of the cerebellum show Purkinje cells. (B) Immunohistochemical analysis of SUMO-1 in cortical neurons and Purkinje cells with hematoxylin counterstaining. Red color labels SUMO-1 and blue labels nuclei. Note the nuclear and perinuclear clustering in TOR samples in contrast to the diffuse cytoplasmic staining in ACR samples. Scale bars show 10 µm. (C) Confocal microscopy showing the subcellular distribution of SUMO-1 in cortical neurons of ACR and TOR. Red: SUMO-1, Blue: nuclei. Scale bars show 10 µm. (D) Confocal microscopy showing the subcellular distribution of PML nuclear bodies in brain sections from ACR and TOR. The far right panels show magnified images of cropped areas in the left panels. Green: PML, Blue: nuclei. Scale bars show 10 µm.
Extensive SUMO-1 and SUMO-2/3 Conjugation Occurs in the Kidney and Liver of Torpid Ground Squirrels
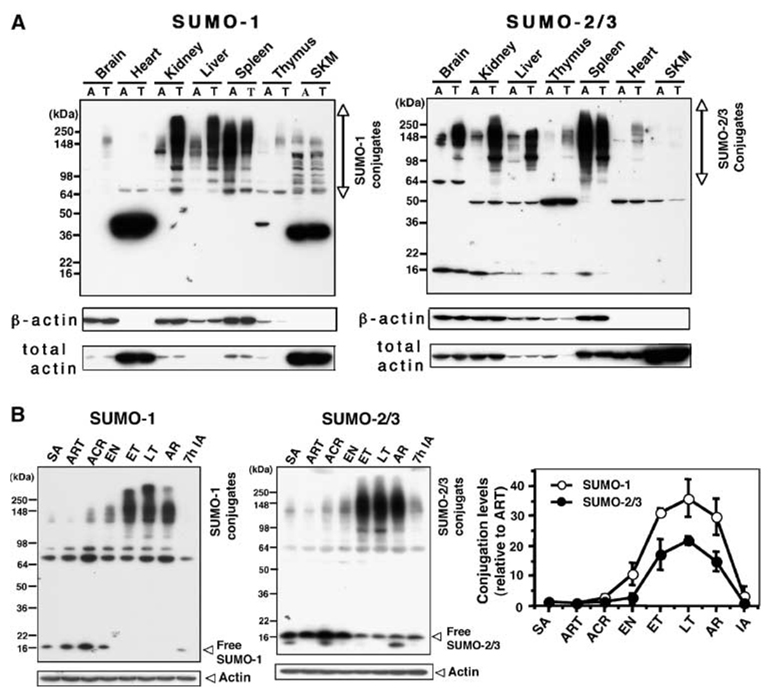
The increase in conjugated SUMO species (higher molecular weight bands) during torpor was even more dramatic in liver and kidney than in the brain (Figure 3A). In heart and skeletal muscle, little or no high molecular weight SUMO conjugation was detected, but relatively low molecular weight (40 to 45 kDa) SUMO-1 conjugates were abundant. The levels of these lower molecular weight conjugates were not changed during torpor, however (Figure 3A). Interestingly, proteins in spleen were heavily SUMOylated even in active animals, and the levels of both SUMO-1 and SUMO-2/3 conjugation decreased during the torpor phase (Figure 3A). Conjugation of both SUMO-1 and SUMO-2/3 in the kidney was similar to brain (Figure 1C) in that the levels began increasing on entrance into torpor, progressively increased throughout the torpor phase and returned to baseline levels by 7 h after interbout arousal (Figure 3B). Conjugation level throughout the torpor phase had a significant umbrella ordering pattern with the peak possibly at the LT (Mack–Wolfe test, SUMO-1: P ≪ 0.01, SUMO-2/3: P ≪ 0.01).
Figure 3.
Distribution of SUMO-1 and SUMO-2/3 conjugates in various tissues from active animals and animals in the torpor phase of the hibernation bout. (A) SUMO-1 or SUMO-2/3 immunoblots of protein extracts from different tissues (as indicated in the figure) from active (A) and torpor (T) phase animals. Actin (both β and total) served as loading controls. Each gel is representative of three independent experiments. SKM: skeletal muscle. (B) Two immunoblots, one of conjugated and free SUMO-1 and one of conjugated and free SUMO-2/3, in the kidney during the phases of the hibernation bout. β-Actin served as a loading control. SA: summer active animals. Densities of bands (conjugates) were normalized by the corresponding actin levels and expressed as relative density (–fold) compared with ART samples (right panel). Data represent the mean±s.d. of three independent experiments.
Small Ubiquitin-Related Modifier Conjugation Levels Correlate with the Expression Levels of Ubc9
Small ubiquitin-related modifier conjugation and deconjugation are dynamic processes in the cell, thus increased SUMOylation occurs from either acceleration (activation) of the conjugating process or inhibition of the deconjugating process. We examined protein expression levels of both the SUMO E2-conjugating enzyme, Ubc9, and one of the SUMO isopeptidases, SENP-1, at various stages of the hibernation bout. As shown in Figure 4, the expression level of Ubc9 coincides with the SUMO conjugation levels in both brain (Figure 1C) and kidney (Figure 3B), but SENP1 levels show no obvious correlation with SUMO conjugation levels in either brain or kidney. The expression level of Ubc9 had a significant umbrella ordering pattern with the peak possibly at the AR stage during torpor (Mack–Wolfe test, brain: P ≪ 0.01, kidney: P ≪ 0.01).
Figure 4.
Expression levels of Ubc9 and SENP1 in the brain and kidney of ground squirrels at various stages of the hibernation bout. Immunoblots of total brain extracts (left panel) or kidney extracts (right panel) from animals in the different phases of the hibernation bout. β-Actin served as a loading control. Densities of bands corresponding to Ubc9 or SENP1 were normalized by actin levels and expressed relative (-fold) to levels of samples from ART (bottom). Data represent the mean±s.d. of three independent experiments.
Hypothermia may be Partially Responsible for the Increased Small Ubiquitin-Related Modifier Conjugation during Hibernation Torpor
We examined whether hypothermia contributes to SUMOylation in torpid animals by anesthetizing nonhibernating ground squirrels in the 4°C to 5°C environmental chamber to lower their body temperature to 24°C, which is equivalent to their body temperature during entrance into torpor. The brains from two out of four such animals showed a modest but detectible increase in protein SUMOylation similar to those from the entrance stage animals (Figure 5A). During torpor in the environmental chamber, the body temperature of ground squirrels falls to about 5°C to 8°C; SUMO conjugation could have been further increased at that temperature. This profound level of body temperature reduction that is characteristic of the torpor phase of hibernation is lethal in most species (including ground squirrels) if induced artificially, which complicates direct testing of SUMOylation at torpor-equivalent temperatures in nonhibernators. We examined the effect of both 24°C and 4°C temperatures on the SUMO conjugation levels in human SHSY5Y cells and found that there was a significant mean difference between 24°C and 4°C temperatures without taking incubation time into account (two-sample t-test, P ≪ 0.0001). As shown in Figure 5B, incubation of these cells at 24°C did increase the SUMO conjugation level and sustained the increase for at least 9 h, but lowering the temperature further to 4°C produced no additional increase of the SUMO conjugation levels. When SHSY5Y cells were subjected to OGD, an in vitro experimental model of ischemia, there existed significant mean differences on the SUMO-1 conjugation levels between two temperatures (37°C and 4°C) regardless of different lengths of OGD (two-sample t-test, P ≪ 0.0001). The SUMO-1 conjugation levels decreased during OGD at 37°C, but cells subjected to OGD at 4°C (similar to the torpor phase of hibernation) showed SUMO conjugation levels that were increased for at least 9 h before showing a trend toward decline by 12 h (Figure 5C). Thus, hypothermia contributes to increased SUMO conjugation during hibernation torpor. In SHSY5Y cells, however, the 24°C level of SUMO conjugation does not increase further in cells exposed to 4°C. It is still not clear whether hypothermia can account for all of the increased SUMOylation during torpor, and effects observed in cell lines may not be an accurate reflection of what is happening in vivo.
The Small Ubiquitin-Related Modifier Conjugation Level Determines the Level of Tolerance to Oxygen/Glucose Deprivation in SHSY5Y Cells
Preconditioning animals or cells by exposure to a near-lethal stress induces transient tolerance to a subsequent severe insult that would otherwise be lethal. We have reported effects of various forms of preconditioning on ischemia tolerance in animals (Tasaki et al, 1997), primary neurons (Liu et al, 2000), and cell lines (Hillion et al, 2005). Here we show that SHSY5Y cells preconditioned by 6 h OGD exposure at 37°C have about a 30% reduction in cell death during severe OGD and that this protection approaches a 50% cell death reduction when the preconditioning is performed at 4°C (Figure 6A). To take into account the baseline cell viability, percent changes of cell death data calculated by using the three averages of percent of cell death from OGD(−) condition at each of the three preconditions were obtained and analysis of cell death data was based on the percent change. There was significant difference in percent change among the three conditions (without PC, PC at 37°C and PC at 4°C) (Kruskal-Wallis test, P = 0.01). A trend analysis via the Jonckheere-Terpstra test indicated that there was a significant decreasing pattern on percent change in order of without PC, 37°C PC, and 4°C PC (P = 0.004).
Figure 6.
In SHSY5Y cells, preconditioning (6 h of OGD) and hypothermia increase SUMOylation; increased SUMOylation greatly protects against OGD and decreased SUMOylation eliminates protection from OGD. (A) Preconditioning SHSY5Y cells at either 37°C or 4°C reduces the percentage of cell death caused by 12 h of OGD exposure. Data represent the mean±s.d. of five independent experiments. (B) Upper panel: Preconditioning at 37°C or 4°C increases SUMO-1 conjugation in SHSY5Y cells; OGD depresses SUMO-1 conjugation in nonpreconditioned cells. Lower panel: Densities of the SUMO-1 conjugated proteins were normalized to actin levels at each time point and expressed as ratios to SUMO-1 conjugated protein level of control cells (0 time point of nonpreconditioned cells). (C) Ubc9 expression by T-Rex-SHSY5Y cell lines that stably expressed Myc-tagged Ubc9 (WT) or Myc-tagged Ubc9 (DN) was tightly regulated by tetracycline (tet). Parent cells are T-Rex-SHSY5Y cells that lack a second plasmid. Actin levels served as a loading control. (D) SUMO-1 conjugation levels in T-Rex-SHSY5Y stable transfectant cells were increased by overexpressed Ubc9 (WT) and were decreased by overexpressed Ubc9 (DN) (Top panel). Small ubiquitin-related modifier-1 conjugation band densities were normalized by the corresponding actin band density and expressed relative to the normalized band density of the parent cells. (E) Effect of overexpression of WT or DN Ubc9 on OGD-induced cell death. T-Rex-SHSY5Y cells stably transfected with Ubc9 (WT) or Ubc9 (DN) along with parent cells were grown in the presence of tetracycline (tet) (for 24 h before PC) and subjected to severe OGD (12 h) without or with preconditioning at either 37°C or 4°C. The cell death was assessed by nuclear staining with Hoechst 33342 and PI followed by flourescence-activated cell sorting analysis. The percentage of dead cells was plotted for each treatment. The data are shown as the mean±s.d. of three independent experiments.
Small ubiquitin-related modifier conjugation levels in SHSY5Y cells increase by preconditioning (6 h OGD) either at 37°C or at 4°C (Figure 5C), but after 24 h reperfusion at 37°C the levels went down close to the control level (Figure 6B, 0 time). However, the SUMOylation levels in preconditioned cells were maintained during severe OGD (12 h) in contrast to a 50% decrease in SHSY5Y cells that have not been preconditioned (Figure 6B). There was a significant difference in SUMO-1 conjugation levels among the three conditions at 12 h (Kruskal–Wallis test, P = 0.003). A trend analysis via the Jonckheere–Terpstra test indicated that there was a significant decreasing pattern on SUMO-1 conjugation in order of without PC, 37°C PC, and 4°C PC (P = 0.001).
To further examine the role of SUMOylation in preconditioning, we established stable transfectants of SHSY5Y cells with a tetracycline-dependent plasmid for overexpression of either a wild type (WT) Ubc9 or a DN Ubc9. The expression levels of Ubc9 (WT) and Ubc9 (DN) were tightly controlled by tetracycline (Figure 6C). Compared with SUMO conjugation levels in the parent cells, transfected cells that overexpressed Ubc9 (WT) increased their protein SUMOylation and transfectants that overexpressed Ubc9 (DN) suppressed their protein SUMOylation (Figure 6D).
Even without preconditioning, SHSY5Y cells overexpressing Ubc9 (WT) were highly resistant to OGD and their increased capacity for SUMOylation raised their level of tolerance to be essentially equivalent the level of tolerance induced by preconditioning; little or no further cytoprotection was achieved by preconditioning at 37°C or by hypothermic preconditioning at 4°C (Figure 6E). The cells that overexpressed Ubc9 (DN) had very little SUMO conjugation and were very sensitive to OGD. In fact, overexpression of Ubc9 (DN) was sufficient to cause cell death in the absence of OGD. Significant differences in percent change among the three cell types were found by implementing the Kruskal–Wallis test: without preconditioning; WT cell group 29%, Parent cell group 44%, DN cell group 51% (P = 0.004); with PC at 37°C, WT group 18%, Parent group 27%, DN group 40% (P = 0.003); PC at 4°C; WT group 16%, Parent group 22%, DN group 41% (P = 0.01). A trend analysis via the Jonckheere–Terpstra test indicated that there was a significant increasing pattern of percent change of cell death without preconditioning (P = 0.0006), with 37°C PC (P = 0.0005), or with 4°C PC (P = 0.001) in the order of Ubc9 (WT), Parent cell, and Ubc9 (DN).
These results indicate that normal levels of SUMO conjugation are required for SHSY5Y cell survival, that SUMOylation undergoes a sustained increase in response to ischemic preconditioning with and without hypothermia, and that increased SUMOylation greatly augments tolerance to OGD in these cells.
Discussion
Hibernation is an adaptive state in which the animal lowers energy consumption, blood flow and body temperature as a means of dealing with food shortage and the energetic cost of defending against seasonal cold. In spite of severe oligemia for periods that can extend to weeks at a time, there is no brain damage or cellular loss in hibernators (reviewed in Carey et al, 2003; Storey, 2003), thus providing an excellent natural model of tolerance to ischemia (Frerichs et al, 1994). A number of molecular mechanisms that regulate this natural state of tolerance have been examined (Chen et al, 2001; Drew et al, 2001; Frerichs et al, 1998; Gentile et al, 1996; Ohtsuki et al, 1998; Zhu et al, 2005), but the full picture is far from clear. In this study, we present data that strongly support SUMO conjugation as a cytoprotective molecular mechanism that is augmented during the profound reduction in blood flow and capacity to deliver oxygen that characterizes hibernation torpor.
We find that protein SUMOylation is markedly elevated in most areas of the brain during the torpor phase of hibernation with a concomitant loss of free SUMO. Small ubiquitin-related modifier conjugation decreases quickly on emergence from torpor indicating a tight linkage of SUMO conjugation with the hibernation process. Although free SUMO is distributed throughout the cell, most of the conjugated SUMO is nuclear, consistent with transcription factors being major targets of SUMOylation (Girdwood et al, 2004).
Hibernation-associated SUMOylation is not, however, restricted to the brain: an even greater SUMO conjugation occurs in liver and kidney tissue. Kidneys and livers from hibernating ground squirrels are reported to be more tolerant to hypothermic storage (as assessed by animal survival after organ transplantation) compared with kidneys and livers taken from normothermic ground squirrels (Green, 2000; Lindell et al, 2005; Storey, 2004). The increased SUMOylation in hibernating ground squirrel tissues may have contributed to the hypothermic storage tolerance of those organs. Unlike other organs we examined, spleens were heavily SUMOylated even in active animals. Since little (or no) analysis of SUMO tissue distribution has been performed, it is unclear whether the distributions noted here are typical of most vertebrate tissues or are restricted to hibernating animals. However, comparison of rat Ubc9 expression levels in various tissues (Golebiowski et al, 2003) showed interesting parallels with SUMO-1 tissue distributions observed in our ground squirrels that is, the highest expression in spleen, relatively high expression in kidney and liver, and low expression in heart and skeletal muscle.
The functional consequence of massive and general SUMOylation of proteins during hibernation torpor is not yet clear, but SUMOylation is known to turn off a wide range of transcription factors (Girdwood et al, 2004) and may contribute to the suppression of gene expression during hibernation (Storey and Storey, 2004). The tumor suppressor PML was originally identified as part of a fusion protein with the retinoic acid receptor (RARa) resulting from a chromosomal translocation that causes acute PML (Kakizuka et al, 1991). Promyelocytic leukemia is essential for the SUMOylation-dependent formation of a nuclear body that also contains many other SUMO-modified proteins including transcription factors, chromatin modifiers, and proteins involved in genomic maintenance (Sternsdorf et al, 1997). Disruption of PML nuclear bodies is associated with changes in cell proliferation, differentiation, and survival (reviewed in Zhong et al, 2000). We surmise that the reported torpor phase-specific nuclear body formation in the hazel dormouse (Malatesta et al, 2001) was due to PML nuclear body formation with accumulation of SUMO conjugates. This suggests that storage of SUMOylated proteins is a role of nuclear body formation during torpor. The structures are proposed to serve as storage/assembly sites for molecules needed for rapid resumption of transcriptional and post-transcriptional activities on arousal from torpor (Malatesta et al, 2001).
The peak of SUMOylation occurs from late torpor to the point of arousal. In stroke patients, extension of brain damage can occur during reperfusion of the ischemic lesion (Hallenbeck and Dutka, 1990). Similarly, the greatest danger of oxidative damage to tissues of a hibernating animal would be at the time of rewarming and renewed activity during arousal (Drew et al, 2002). The fact that SUMOylation peaks on arousal may relate to its having cytoprotective effects. This reversible modification of proteins may contribute to the ability of hibernators to lower metabolic rates during the torpor phase, but retain the ability to resume full activity on arousal without any tissue damage.
We find that the expression levels of Ubc9, the single E2-conjugating enzyme in the SUMO pathway, are closely correlated with SUMO conjugation levels both in the brain and the kidney during hibernation, and that Ubc9 expression levels are also critically related to the capacity of SHSY5Y cells to survive ischemic insults. Ubc9 is reported to be essential for viability of higher eukaryotic cells; Ubc9 deficient mouse embryos die at the early postimplantation stage (Nacerddine et al, 2005) and depletion of Ubc9 in chicken DT40 cells results in death by apoptosis (Hayashi et al, 2002). The profound changes of SUMO conjugation levels in hibernation may not be fully explained by the modest changes of Ubc9 levels noted here, however. Unlike Ubc9, the relationship between a SUMO isopeptidase, SENP1, and SUMOylation levels showed no correlation in the tissues examined. Ubc9 is the only SUMO E2-conjugating enzyme, but there are at least six SENPs known in mammals (Melchior et al, 2003; Yeh et al, 2000). Whether any of these SENPs is involved in elevating the level of SUMOylation during hibernation remains unclear.
SUMOylation provides impressive cytoprotection in SHSY5Y cell OGD. Suppression of the capacity to SUMOylate proteins eliminates SHSY5Y cell resistance to OGD and nullifies cytoprotection by preconditioning with or without hypothermia in these cells. The fact that the percent increase in OGD-induced cell death appears to be somewhat less in DN cells and DN cells do not respond to preconditioning needs to be addressed. These cells were already very sick before exposure to OGD. This is due to their inability to maintain normal levels of SUMO conjugation to proteins as a result of their overexpression of the DN mutation of the critical SUMO conjugating enzyme, Ubc9. After the cultures were exposed to OGD, most of the DN cells as measured by our assay were dead. The cell death assay we used is not very accurate above 80% cell death, so the cell death by OGD in DN group might have been saturated such that the actual cell death was more than 80%. The DN group is not made more resistant to OGD by preconditioning, presumably because it cannot turn on the SUMOylation system. We should also emphasize that the observation that preconditioning fails to enhance tolerance to OGD in DN cells is consistent with a vital role for SUMOylation in preconditioning of SHSY5Y cell cultures. The inhibition of endogenous Ubc9 by overexpression of the DN protein is toxic and apparently did prevent the protection otherwise afforded by preconditioning. This finding indicates that SUMOylation is so important for cell survival that preconditioning cannot overcome its absence or compensate for its absence. The three findings that cells overexpressing Ubc9 WT were highly resistant to OGD, that the tolerance was not enhanced by preconditioning in these cells, and that preconditioning serves to maintain normal levels of SUMOylation (Figure 6B) also support the idea that the SUMOylation is importantly involved in preconditioning-induced tolerance.
Although further studies are indicated to fully delineate the specific SUMOylation mechanisms that confer hypothermic cytoprotection, our finding that SUMO conjugation increases during hypothermia and is essential for cellular resistance to OGD during preconditioning with or without hypothermia has clinical relevance. Our study suggests decreased body temperature may be a primary mechanism for increasing SUMOylation in hibernation torpor, and SUMO conjugation may be an underling mechanism for hypothermia-induced tolerance to ischemia. Hypothermia is currently under clinical investigation as a potential therapeutic approach to acute ischemic stroke (De Georgia et al, 2004). Our findings also point the way to a means to increase our understanding of the molecular mechanisms involved in preconditioning-induced ischemic tolerance by examining the SUMOylated protein profile that supports ischemia resistance. Finally, the data suggest that elevation of SUMO conjugation levels may be an appropriate target for drug discovery in the cerebrovascular disease field.
Acknowledgements
We thank Mary Dasso for providing various antibodies and DNA constructs and helpful comments, Dragan Maric for FACS analysis and Christl Ruetzler for preparing tissue sections. We also thank Ferhan Ayaydin and Maria Spatz for critical reading of the manuscript.
This research was supported by the Intramural Research Program of the NINDS/NIH.
References
- Azuma Y, Arnaoutov A, Dasso M. SUMO-2/3 regulates topoisomerase II in mitosis. J Cell Biol. 2003;163:477–487. doi: 10.1083/jcb.200304088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Chen Y, Matsushita M, Nairn AC, Damuni Z, Cai D, Frerichs KU, Hallenbeck JM. Mechanisms for increased levels of phosphorylation of elongation factor-2 during hibernation in ground squirrels. Biochemistry. 2001;40:11565–11570. doi: 10.1021/bi010649w. [DOI] [PubMed] [Google Scholar]
- De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, Koroshetz WJ, Rordorf G, Warach S. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63:312–317. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Drew KL, Rice ME, Kuhn TB, Smith MA. Neuroprotective adaptations in hibernation: therapeutic implications for ischemia–reperfusion, traumatic brain injury and neurodegenerative diseases. Free Radic Biol Med. 2001;31:563–573. doi: 10.1016/s0891-5849(01)00628-1. [DOI] [PubMed] [Google Scholar]
- Drew KL, Toien O, Rivera PM, Smith MA, Perry G, Rice ME. Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133:483–492. doi: 10.1016/s1532-0456(02)00118-7. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab. 1998;18:168–175. doi: 10.1097/00004647-199802000-00007. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to ‘cerebral ischemia’. J Cereb Blood Flow Metab. 1994;14:193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Smith CB, Brenner M, DeGracia DJ, Krause GS, Marrone L, Dever TE, Hallenbeck JM. Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci USA. 1998;95:14511–14516. doi: 10.1073/pnas.95.24.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile NT, Spatz M, Brenner M, McCarron RM, Hallen-beck JM. Decreased calcium accumulation in isolated nerve endings during hibernation in ground squirrels. Neurochem Res. 1996;21:947–954. doi: 10.1007/BF02532345. [DOI] [PubMed] [Google Scholar]
- Girdwood DW, Tatham MH, Hay RT. SUMO and transcriptional regulation. Semin Cell Dev Biol. 2004;15:201–210. doi: 10.1016/j.semcdb.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Golebiowski F, Szulc A, Sakowicz M, Szutowicz A, Pawelczyk T. Expression level of Ubc9 protein in rat tissues. Acta Biochim Pol. 2003;50:1065–1073. [PubMed] [Google Scholar]
- Green C. Mammalian hibernation: lessons for organ preparation? Cryo Lett. 2000;21:91–98. [PubMed] [Google Scholar]
- Hallenbeck JM, Dutka AJ. Background review and current concepts of reperfusion injury. Arch Neurol. 1990;47:1245–1254. doi: 10.1001/archneur.1990.00530110107027. [DOI] [PubMed] [Google Scholar]
- Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Seki M, Maeda D, Wang W, Kawabe Y, Seki T, Saitoh H, Fukagawa T, Yagi H, Enomoto T. Ubc9 is essential for viability of higher eukaryotic cells. Exp Cell Res. 2002;280:212–221. doi: 10.1006/excr.2002.5634. [DOI] [PubMed] [Google Scholar]
- Hillion JA, Takahashi K, Maric D, Ruetzler C, Barker JL, Hallenbeck JM. Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab. 2005;25:154–162. doi: 10.1038/sj.jcbfm.9600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizuka A, Miller WH, Jr, Umesono K, Warrell RP, Jr, Frankel SR, Murty VV, Dmitrovsky E, Evans RM. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Lapenta V, Chiurazzi P, van der Spek P, Pizzuti A, Hanaoka F, Brahe C. SMT3A, a human homologue of the S. cerevisiae SMT3 gene, maps to chromosome 21 qter and defines a novel gene family. Genomics. 1997;40:362–366. doi: 10.1006/geno.1996.4556. [DOI] [PubMed] [Google Scholar]
- Lehmann EL. Nonparametrics: statistical methods based on ranks. San Francisco: Holden-Day; 1975. [Google Scholar]
- Lindell SL, Klahn SL, Piazza TM, Mangino MJ, Torrealba JR, Southard JH, Carey HV. Natural resistance to liver cold ischemia–reperfusion injury associated with the hibernation phenotype. Am J Physiol Gastrointest Liver Physiol. 2005;288:G473–G480. doi: 10.1152/ajpgi.00223.2004. [DOI] [PubMed] [Google Scholar]
- Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am J Physiol Cell Physiol. 2000;278:C144–C153. doi: 10.1152/ajpcell.2000.278.1.C144. [DOI] [PubMed] [Google Scholar]
- Mack GA, Wolfe DA. K-sample rank tests for umbrella alternatives. J Am Stat Assoc. 1981;76:175–181. [Google Scholar]
- Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- Malatesta M, Luchetti F, Marcheggiani F, Fakan S, Gazzanelli G. Disassembly of nuclear bodies during arousal from hibernation: an in vitro study. Chromosoma. 2001;110:471–477. doi: 10.1007/s004120100166. [DOI] [PubMed] [Google Scholar]
- Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol. 1996;135:1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F, Schergaut M, Pichler A. SUMO: ligases, isopeptidases and nuclear pores. Trends Biochem Sci. 2003;28:612–618. doi: 10.1016/j.tibs.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Muller S, Hoege C, Pyrowolakis G, Jentsch S. SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell Biol. 2001;2:202–210. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- Nacerddine K, Lehembre F, Bhaumik M, Artus J, Cohen-Tannoudji M, Babinet C, Pandolfi PP, Dejean A. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell. 2005;9:769–779. doi: 10.1016/j.devcel.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Ohtsuki T, Jaffe H, Brenner M, Azzam N, Azzam R, Frerichs KU, Hallenbeck JM. Stimulation of tyrosine phosphorylation of a brain protein by hibernation. J Cereb Blood Flow Metab. 1998;18:1040–1045. doi: 10.1097/00004647-199809000-00014. [DOI] [PubMed] [Google Scholar]
- Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem. 2000;275:6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- Saitoh H, Pu R, Cavenagh M, Dasso M. RanBP2 associates with Ubc9p and a modified form of Ran-GAP1. Proc Natl Acad Sci USA. 1997;94:3736–3741. doi: 10.1073/pnas.94.8.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternsdorf T, Jensen K, Will H. Evidence for covalent modification of the nuclear dot-associated proteins PML and Sp100 by PIC1/SUMO-1. J Cell Biol. 1997;139:1621–1634. doi: 10.1083/jcb.139.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KB, Storey JM. Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev Cambr Philos Soc. 2004;79:207–233. doi: 10.1017/s1464793103006195. [DOI] [PubMed] [Google Scholar]
- Storey KB. Mammalian hibernation. Transcriptional and translational controls. Adv Exp Med Biol. 2003;543:21–38. [PubMed] [Google Scholar]
- Storey KB. Cold ischemic organ preservation: lessons from natural systems. J Invest Med. 2004;52:315–322. doi: 10.1136/jim-52-05-31. [DOI] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–270. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Yeh ET, Gong L, Kamitani T. Ubiquitin-like proteins: new wines in new bottles. Gene. 2000;248:1–14. doi: 10.1016/s0378-1119(00)00139-6. [DOI] [PubMed] [Google Scholar]
- Zhong S, Salomoni P, Pandolfi PP. The transcriptional role of PML and the nuclear body. Nat Cell Biol. 2000;2:E85–E90. doi: 10.1038/35010583. [DOI] [PubMed] [Google Scholar]
- Zhu X, Smith MA, Perry G, Wang Y, Ross AP, Zhao HW, Lamanna JC, Drew KL. MAPKs are differentially modulated in arctic ground squirrels during hibernation. J Neurosci Res. 2005;80:862–868. doi: 10.1002/jnr.20526. [DOI] [PubMed] [Google Scholar]