Abstract
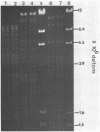
The cryptic plasmid pRUT41 from Zymomonas mobilis was examined for its biological properties. This plasmid was found to be conjugally transferred from Z. mobilis CP4 to Escherichia coli BM21 and to carry genes for antibiotic resistance (gentamicin, kanamycin, and streptomycin). Covalently closed circular plasmid DNA was isolated from eight transconjugants of E. coli BM21. These plasmids were identical in mobility on agarose gels and exhibited the same restriction patterns as the native pRUT41 plasmid isolated from Z. mobilis. The plasmid location of the antibiotic resistance genes was further confirmed by transforming E. coli BM21 with isolated pRUT41 plasmid from strain CP4 and with plasmids from the transconjugants of BM21. Resistance to streptomycin, kanamycin, and gentamicin was tightly linked and transferred together in all cases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S. The ecology of transferable drug resistance in the enterobacteria. Annu Rev Microbiol. 1968;22:131–180. doi: 10.1146/annurev.mi.22.100168.001023. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMOSS R. D., GIBBS M. Ethanol formation in Pseudomonas lindneri. Arch Biochem Biophys. 1951 Dec;34(2):478–479. doi: 10.1016/0003-9861(51)90028-8. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H., Dunn G. B., Pinkerton T., Rose K., Walia S. K. Colicin activity and abortive infection of T5 bacteriophage in Escherichia coli (ColIb). J Virol. 1981 Mar;37(3):916–921. doi: 10.1128/jvi.37.3.916-921.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS M., DEMOSS R. D. Anaerobic dissimilation of C14-labeled glucose and fructose by Pseudomonas lindneri. J Biol Chem. 1954 Apr;207(2):689–694. [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Nofsinger G. W., Bothast R. J. Ethanol production by Zymomonas mobilis and Saccharomyces uvarum on aflatoxin-contaminated and ammonia-detoxified corn. Can J Microbiol. 1981 Feb;27(2):162–167. doi: 10.1139/m81-026. [DOI] [PubMed] [Google Scholar]
- Skotnicki M. L., Lee K. J., Tribe D. E., Rogers P. L. Comparison of ethanol production by different zymomonas strains. Appl Environ Microbiol. 1981 Apr;41(4):889–893. doi: 10.1128/aem.41.4.889-893.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotnicki M. L., Tribe D. E., Rogers P. L. R-Plasmid Transfer in Zymomonas mobilis. Appl Environ Microbiol. 1980 Jul;40(1):7–12. doi: 10.1128/aem.40.1.7-12.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes H. W., Dally E. L., Yablonsky M. D., Eveleigh D. E. Comparison of plasmids in strains of Zymomonas mobilis. Plasmid. 1983 Mar;9(2):138–146. doi: 10.1016/0147-619x(83)90016-1. [DOI] [PubMed] [Google Scholar]
- Swings J., De Ley J. The biology of Zymomonas. Bacteriol Rev. 1977 Mar;41(1):1–46. doi: 10.1128/br.41.1.1-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia S. K., Chugh T. D., Sharma K. B. Prevalence of R-plasmid in Klebsiella pneumoniae. Indian J Med Res. 1980 Jan;71:42–45. [PubMed] [Google Scholar]