Abstract
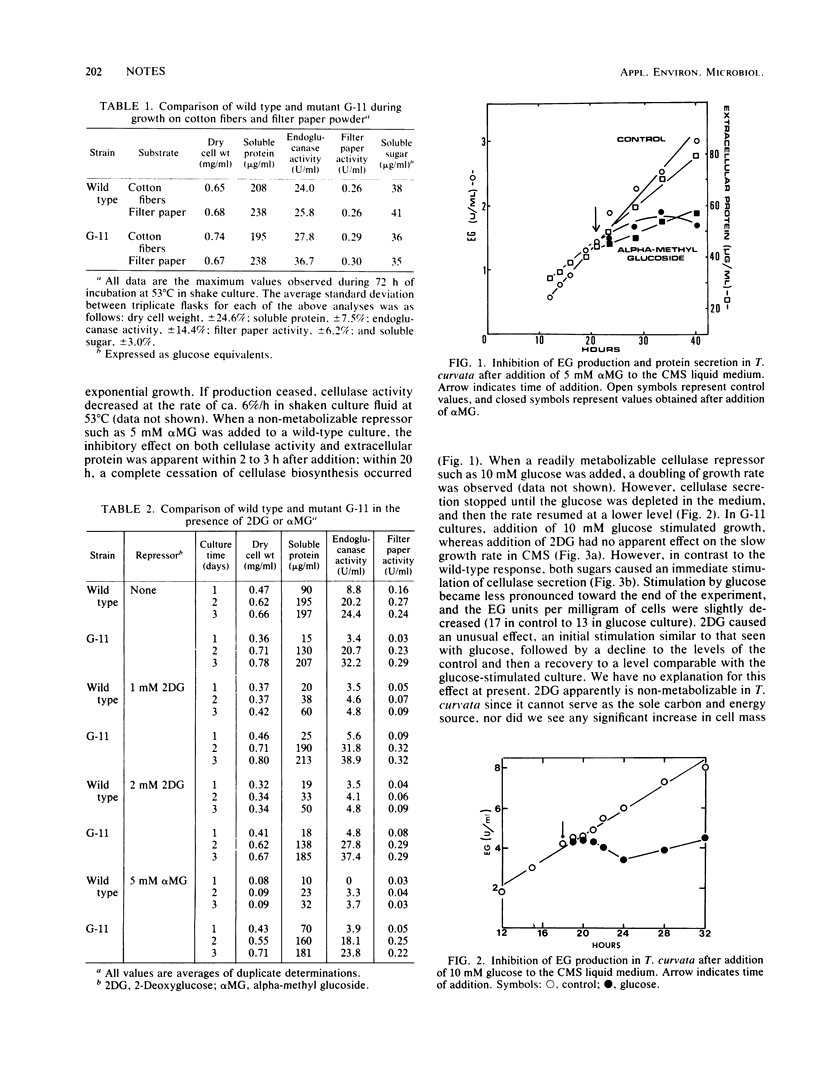
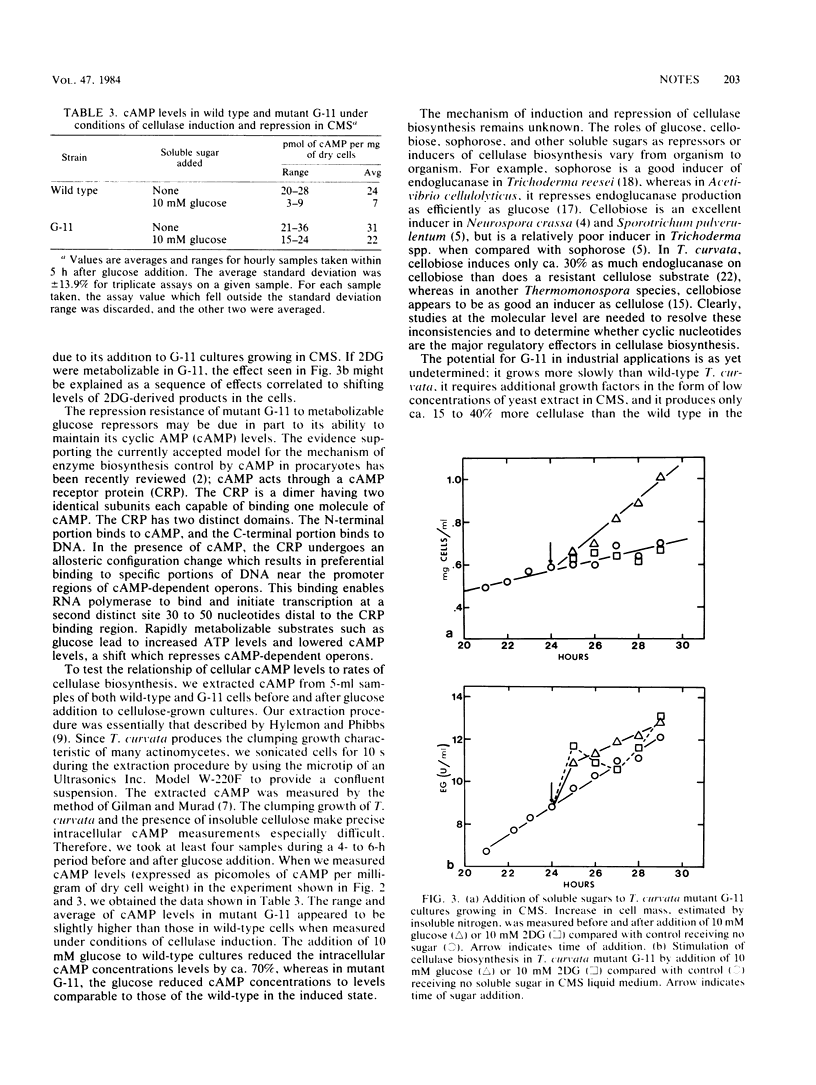
A catabolite repression-resistant mutant of the thermophilic actinomycete Thermomonospora curvata was obtained by treatment with ethyl methanesulfonate and UV light. Cellulase biosynthesis was undiminished by glucose, 2-deoxyglucose, or alpha-methyl glucoside, which are potent repressors in the wild type. Intracellular cyclic AMP levels were higher in the mutant in both the absence and the presence of repressors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Eberhart B. M., Beck R. S., Goolsby K. M. Cellulase of Neurospora crassa. J Bacteriol. 1977 Apr;130(1):181–186. doi: 10.1128/jb.130.1.181-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K. E., Hamp S. G. Regulation of Endo-1,4-beta-glucanase production in Sporotrichum pulverulentum. Eur J Biochem. 1978 Sep 15;90(1):183–190. doi: 10.1111/j.1432-1033.1978.tb12589.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G., Murad F. Assay of cyclic nucleotides by receptor protein binding displacement. Methods Enzymol. 1974;38:49–61. doi: 10.1016/0076-6879(74)38010-x. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Evidence against the presence of cyclic AMP and related enzymes in selected strains of Bacteroides fragilis. Biochem Biophys Res Commun. 1974 Sep 9;60(1):88–95. doi: 10.1016/0006-291x(74)90176-4. [DOI] [PubMed] [Google Scholar]
- MANDELS M., REESE E. T. Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J Bacteriol. 1957 Feb;73(2):269–278. doi: 10.1128/jb.73.2.269-278.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandels M., Andreotti R., Roche C. Measurement of saccharifying cellulase. Biotechnol Bioeng Symp. 1976;(6):21–33. [PubMed] [Google Scholar]
- Nisizawa T., Suzuki H., Nisizawa K. Catabolite repression of cellulase formation in Trichoderma viride. J Biochem. 1972 Jun;71(6):999–1007. doi: 10.1093/oxfordjournals.jbchem.a129872. [DOI] [PubMed] [Google Scholar]
- Saddler J. N., Khan A. W., Martin S. M. Regulation of cellulase synthesis in Acetivibrio cellulolyticus. Microbios. 1980;28(112):97–106. [PubMed] [Google Scholar]
- Sternberg D., Mandels G. R. Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol. 1979 Sep;139(3):761–769. doi: 10.1128/jb.139.3.761-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg D., Mandels G. R. Regulation of the cellulolytic system in Trichoderma reesei by sophorose: induction of cellulase and repression of beta-glucosidase. J Bacteriol. 1980 Dec;144(3):1197–1199. doi: 10.1128/jb.144.3.1197-1199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart B. J., Leatherwood J. M. Derepressed synthesis of cellulase by Cellulomonas. J Bacteriol. 1976 Nov;128(2):609–615. doi: 10.1128/jb.128.2.609-615.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppok W., Rapp P., Wagner F. Formation, Location, and Regulation of Endo-1,4-beta-Glucanases and beta-Glucosidases from Cellulomonas uda. Appl Environ Microbiol. 1982 Jul;44(1):44–53. doi: 10.1128/aem.44.1.44-53.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzenberger F. J. Cellulolytic activity of Thermomonospora curvata: nutritional requirments for cellulase production. Appl Microbiol. 1972 Jul;24(1):77–82. doi: 10.1128/am.24.1.77-82.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzenberger F. J. Cellulolytic activity of Thermomonospora curvata: optimal assay conditions, partial purification, and product of the cellulase. Appl Microbiol. 1972 Jul;24(1):83–90. doi: 10.1128/am.24.1.83-90.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


