Abstract
The Gga proteins represent a family of ubiquitously expressed clathrin adaptors engaged in vesicle budding at the tubular endosomal network/trans Golgi network. Their membrane recruitment is commonly thought to involve interactions with Arf and signals in cargo through the so-called VHS domain. For yeast Gga proteins, however, partners binding to its VHS domain have remained elusive and Gga localization does not absolutely depend on Arf. Here, we demonstrate that yeast Gga recruitment relies on a network of interactions between the scaffold Ysl2p/Mon2p, the small GTPase Arl1p, and the flippase Neo1p. Deletion of either YSL2 or ARL1 causes mislocalization of Gga2p, whereas a neo1-69 mutant accumulates Gga2p on aberrant structures. Remarkably, Ysl2p directly interacts with human and yeast Ggas through the VHS domain, and binding to Gga proteins is also found for the human Ysl2p orthologue hMon2. Thus, Ysl2p represents an essential, evolutionarily conserved member of a network controlling direct binding and membrane docking of Ggas. Because activated Arl1p is part of the network that binds Gga2p, Arf and Arf-like GTPases may interact in a regulatory cascade.
Keywords: Arf family, clathrin coat, endosomes, flippase, membrane traffic
Introduction
Vesicular trafficking provides a continuous exchange of proteins and lipids between membranous compartments in eukaryotic cells. Two major cellular compartments implicated in sorting within the secretory and endocytic pathways are the trans Golgi network (TGN) and the tubular endosomal network (TEN). They represent a ‘membrane continuum' that shares and exchanges a highly related transport machinery to support the complex sorting functions (Bonifacino and Rojas, 2006). During the selective packaging of different cargo, clathrin and its associated adaptor proteins (APs) provide the central scaffold for the formation and stabilization of various budding intermediates. Because clathrin does not bind directly to lipids, adaptors are needed to anchor the clathrin coat to the budding membrane through association with lipids and cytoplasmic domains of cargo. The classes of clathrin adaptors that participate in budding from the TGN and the TEN are AP-1 and AP-3, members of the heterotetrameric APs, Gga proteins and epsin-like proteins (Bonifacino, 2004; Robinson, 2004). AP adaptor comprise two large subunits (γ and β1), a medium-sized (μ) and a small subunit (σ). Gga proteins are monomeric adaptors characterized by a modular organization. The N-terminal VHS domain binds to sorting signals in cargo proteins, the central GAT domain interacts with Arf and ubiquitin-modified proteins. The hinge region contains clathrin-binding domains and connects to the C-terminal ear domain homologous to that of γ-adaptin of AP-1, which can bind to accessory proteins. Epsin-like proteins, characterized by an ENTH (epsin N-terminal homology) domain, are able to induce membrane curvature on their own, and their interplay with clathrin is thought to stabilize the curved shape of emerging buds.
Coat assembly starts by activation of the ADP ribosylation factor (ARF) family of small GTPases, including the well-characterized Arf1 and Arf6 proteins. Arf1/Arf6 regulate the recruitment of COPI and most clathrin coats to Golgi and endosomal membranes (Bonifacino and Rojas, 2006). The engagement of Arf1 in numerous interactions with effector molecules is far from being understood. The broad range of functions is reflected by the fact that yeast Arf1p may be regulated by at least four GEFs and six putative GAPs. Arf1 communicates with many more effector molecules and thereby regulates the formation of budding structures involved in multiple transport steps by the activation of several adaptors. The fundamental question whether and how Arf1 manages all of these divergent reactions still remains to be solved. In addition to Arfs, a large number of Arf-like (Arl) proteins exist in all eukaryotes. This Arl subgroup has been defined based on sequence and functional relatedness (Kahn, 1995). One of the most highly conserved Arl proteins is Arl1. Initially, it has been localized to the TGN in mammals and yeast (Rosenwald et al, 2002; Lu and Hong, 2003), where it is best known for its role in the membrane recruitment of the GRIP-domain golgins (Lu and Hong, 2003; Panic et al, 2003; Setty et al, 2003). Yet in addition to postulated unique roles for Arf1 and Arl1, there are several clues for common binding partners and a functional interplay between Arf/Arl family members (van Valkenburgh et al, 2001; Lu and Hong, 2003).
In addition to its function at the TGN, an expanded role for Arl1 in the endocytic system is evident from endocytosis defects caused by deletion of ARL1 in yeast (Jochum et al, 2002; Rosenwald et al, 2002). Here, Arl1p has been shown to cooperate with Neo1p and Ysl2p. Neo1p is a member of the Drs2 family of P-type ATPases with proposed aminophospholipid translocase activity (Hua and Graham, 2003; Wicky et al, 2004). Ysl2p, also termed Mon2p, is homologous to two subgroups of large Sec7 Arf GEFs, the GBF and BIG families (Jochum et al, 2002; Efe et al, 2005; Gillingham et al, 2006; Ramaen et al, 2007). Both, Ysl2p and Neo1p localize to endosomal and TGN membranes and reveal biochemical and genetic interactions with each other (Jochum et al, 2002; Wicky et al, 2004; Efe et al, 2005). Moreover, Arl1p displays biochemical and genetic interactions with Ysl2p and Neo1p. Among all Arf and Arl family members, Arl1p suppresses various defects associated with Δysl2 cells when overexpressed (Jochum et al, 2002), and it physically interacts with the N terminus of Ysl2p (Jochum et al, 2002). Furthermore, the deletion of ARL1 suppresses conditional mutants in the essential NEO1 gene (Wicky et al, 2004). Our favoured hypothesis to explain this rescue is that suppression disrupts an otherwise detrimental interaction of mutant Neo1p with Arl1p and/or interacting partners. Such assemblies may impair the process of membrane trafficking within the TEN/TGN causing growth inhibition in the neo1 mutants at non-permissive temperature. Despite remaining questions, these studies have established that Ysl2p, Arl1p, and Neo1p closely cooperate in a functional network during vesicle formation and organelle biogenesis in the endosomal system (Wicky et al, 2004).
In this study, we tested the hypothesis whether the Ysl2p–Arl1p–Neo1p network might be implicated in coat recruitment at the TEN/TGN interface. Using a variety of independent approaches, we identified the monomeric Gga adaptors in yeast and human cells as essential binding partners of this network, in particular the scaffold Ysl2p/hMon2. Notably, the direct interaction between Ysl2p/hMon2 and Gga proteins is mediated by their VHS domain previously implicated exclusively in cargo recognition in mammalian cells. Moreover, Gga2p was identified as a novel effector of GTP–Arl1p.
Results
Deletion of GGA2 suppresses the temperature-sensitive neo1-69 mutant
To identify further components interacting with the Neo1p–Ysl2p–Arl1p network, we analysed whether the deletion of putative Arl1p effectors could suppress the temperature sensitivity of neo1-69 cells. In addition to defects in endocytosis and vacuole biogenesis, this mutant exhibits an aberrant morphology of endosomal structures (Wicky et al, 2004; see also below). Because of similarities between Arl1p and classical Arfs (Pasqualato et al, 2002) and the well-known role of Arf1p in coat assembly during vesicle formation, we focused on the β subunits of AP-1 and AP-3 known to be required for AP activity and trafficking from the TGN/TEN. Thus, APL2 (β1 of AP-1) and APL6 (β3 of AP-3) knockouts, respectively, were combined with the neo1-69 allele. Similarly, deletions of GGA1 and GGA2, each encoding monomeric adaptors with function in clathrin coats (Costaguta et al, 2001), were individually tested in combination with the neo1-69 allele.
At 25°C, all double mutants grew similarly and no genetic interaction between neo1-69 and any of the gene knockouts for the APs or their β subunits was observed (Figure 1A). At 37°C, the non-permissive temperature for neo1-69 cells, deletion of GGA2 rescued the growth defect of the neo1-69 mutant. This suppression was comparable to that caused by deletion of ARL1 (Figure 1A; Wicky et al, 2004). Consistent with the role of activated Arl3p in Arl1p recruitment (Panic et al, 2003; Setty et al, 2003), deletion of ARL3 also caused suppression of the neo1-69 growth defect to an extent comparable to that caused by loss of either ARL1 or GGA2. The deletion of APL2 (AP-1 β chain) suppressed the temperature sensitivity of neo1-69 cells; however, the effect was less pronounced. Similarly, the deletion of APL6 (AP-3 β chain) and of ARF1 caused a slight rescue of the temperature-sensitive growth of neo1-69 cells (Figure 1A and B). Notably, loss of GGA1 showed no effect on growth of neo1-69 mutant cells at 37°C (Figure 1A). Given the striking neo1-69–GGA2 genetic interaction, this result was surprising although it provided a useful internal control for specificity. However, similar differential effects of individual GGA1 and GGA2 deletions in combination with loss of clathrin were previously reported and ascribed to differences in protein abundance rather than function (Costaguta et al, 2001). In agreement to that, our strain background contains a 10-fold excess of endogenous Gga2p compared with Gga1p (data not shown), likely explaining why loss of Gga1p does not compensate the detrimental effect of the Neo1-69p allele.
Figure 1.
The neo1-69 mutant can be suppressed by gene deletions of adaptor proteins. (A) Genetic interaction between neo1-69 and deletions of endosomal adaptors. Dilution series of cells of the indicated strains grown at 37°C and 25°C. (B) Genetic interaction between neo1-69 and deletions of endosomal small GTPases. Dilution series of cells as described in (A).
We also tested whether the loss of two Ypt small GTPases essential for trafficking within the endocytic system, Ypt51p and Ypt7p, could suppress the growth defect of neo1-69 cells. However, this was not the case. Therefore, loss of critical endocytic Ypts participating in more distal steps of vesicle biogenesis such as tethering, docking, and fusion, appears not to be detrimental in combination with Neo1-69p.
Expression of Neo1-69p causes abnormal accumulation of Gga2p
To evaluate how loss of Gga2p could cause suppression of neo1-69 cells, we analysed the subcellular distribution of Gga2p in neo1-69 cells relative to wild type. Wild-type endosomes display a complex tubulo-vesicular morphology due to participation in various sorting reactions (Bonifacino and Rojas, 2006). Expression of Neo1-69p causes a drastic alteration of endosomal morphology, as demonstrated by the accumulation of membrane elongations, multivesicular and ring-like tubular structures, and affects the localization and stability of Arl1p and Ysl2p (Wicky et al, 2004). In the neo1-69 mutant, the aberrantly shaped membrane structures may represent enhanced budding intermediates that emanate from the TEN due to impaired Neo1p function (Wicky et al, 2004).
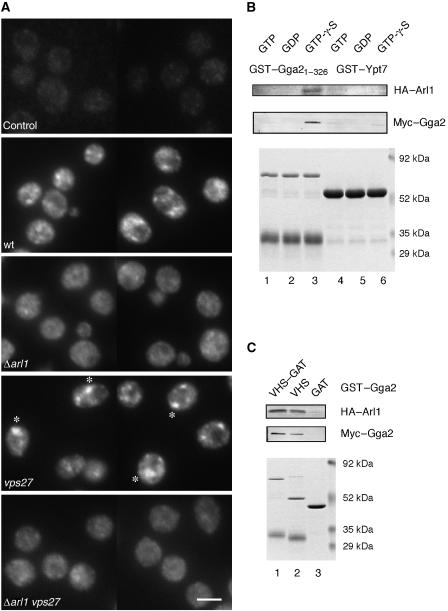
A functional version of Gga2p C-terminally tagged with an HA epitope was expressed from the chromosome and indirect immunofluorescence was performed. In wild-type cells, HA–Gga2p was detected diffusely in the cytoplasm and on multiple small, faintly stained dotted structures likely representing the TGN and endosomes (Figures 2 and 3A; Boman et al, 2002). The somewhat fuzzy appearance of the foci, usually more than five, was typical for the Gga2p staining. In the neo1-69 strain, however, the HA–Gga2p-positive structures were more prominent and appeared brighter and larger. Quantitation showed that 18% of the neo1-69 cells (n=385) exhibited 1–3 bright, large structures, whereas only 3% of wild-type cells (n>500) showed this pattern (Figure 2). This effect is similar to what has been observed in neo1-69 cells about the localization of HA–Arl1p and HA–Ysl2p (Wicky et al, 2004). Examples of neo1-69 cells with such aberrant structures positive for HA–Arl1p are shown for comparison (Figure 2).
Figure 2.
Neo1-69p affects the subcellular distribution of HA–Gga2p. Untagged wild-type cells (control), wild-type (wt) cells expressing HA–Gga2p, and neo1-69 cells expressing either HA–Gga2p or HA–Arl1p were grown at 25°C, fixed, and stained with an anti-HA antibody; *enlarged, distinctive HA–Gga2p spots with an average fluorescence intensity 2.1-fold above values obtained for the same area in wt cells (for each strain, values obtained from 35 spots were averaged). Bar, 5 μm.
Figure 3.
Loss of Ysl2p severely affects the subcellular distribution of HA–Gga2p and HA–Gga1p. Indirect immunofluorescence was performed with wild-type (wt) and Δysl2 cells expressing either HA–Gga2p (A) or HA–Gga1p (C) as described in Figure 2. Relative to HA–Gga2p, images of HA–Gga1p staining were obtained with three-fold longer exposure time. (B) Cell extracts of wt and Δysl2 cells expressing HA–Gga2p or HA–Gga1p were analysed by immunoblotting with an anti-HA and anti-PGK antibody. (D) Wild-type and Δysl2 cells expressing plasmid-encoded Myc–Tlg1p were stained with an anti-c-Myc antibody. (E) Δysl2 cells transformed with empty vector or the indicated plasmids were stained for HA–Gga2p. Bar, 5 μm.
Analysing the distribution of clathrin light chain (Clc1p), tagged N-terminally with an HA epitope to preserve functionality, revealed that it was not notably affected in neo1-69 cells (data not shown). Given the association of clathrin with various budding structures, alterations of only a subset of them may however be difficult to identify.
Loss of Ysl2p severely perturbs the localization of Gga2p
Because of Ysl2p's close biochemical and genetic links to Arl1p (Jochum et al, 2002) and its proposed role as scaffolding protein (Efe et al, 2005; Gillingham et al, 2006), we analysed whether Ysl2p may be implicated in the association of Gga2p with membranes. Remarkably, indirect immunofluorescence revealed that the YSL2 deletion caused an overall loss of HA–Gga2p staining (Figure 3A), indicating that Ysl2p executes a central role for the correct targeting of this clathrin adaptor. The cellular levels of HA–Gga2p in wild-type and Δysl2 cells were however similar (Figure 3B), thus excluding the possibility of alterations in protein expression. When we examined the distribution of HA–Gga2p after fractionation of lysed cells into cytosol and membranes, we detected no significant difference between wild-type and Δysl2 cells (data not shown). Cell lysis may however affect moderate- and low-affinity interactions of Ggas, possibly masking the clear differences revealed with fixed cells.
Loss of Ysl2p also affected the distribution of HA–Gga1p. In wild-type cells, HA–Gga1p was detected in punctate structures. The foci appeared sharper than those positive for HA–Gga2p, but the signal intensity was weaker consistent with the lower abundance of Gga1p (Figure 3C). Importantly, in Δysl2 cells the HA–Gga1p localization became diffuse (Figure 3C), even though the levels of HA–Gga1p were unaffected (Figure 3B). Thus, the absence of Ysl2p has an impact on the localization of both Gga1p and Gga2p.
To exclude the possibility of a generalized mislocalization and/or trafficking of resident TGN/TEN marker proteins, we determined the staining pattern of Myc–Tlg1p, a SNARE family member of the TGN/TEN that colocalizes with Ysl2p and Neo1p (Wicky et al, 2004). However, in Δysl2 cells the staining pattern of Myc–Tlg1p was unaffected (Figure 3D), indicating that the loss of YSL2 does not have pleiotropic effects on membrane trafficking within the TGN/TEN.
We recently demonstrated that the specific overexpression of ARL1 can suppress various defects of Δysl2 cells, including impaired growth, endocytosis, and vacuole biogenesis (Jochum et al, 2002). Another suppressor of the Δysl2 mutant, NEO1, is able to suppress the Δysl2-associated defects even after expression from a low-copy CEN plasmid. Consistent with that and the proposed cooperation among Ysl2p, Arl1p, and Neo1p, expression of CEN NEO1 in Δysl2 cells suppressed the impaired localization of Gga2p, as it re-established the typical Gga2p staining with multiple foci (Figure 3E). Similarly, ARL1 overexpression was able to fully restore the HA–Gga2p localization to dotted structures (Figure 3E), whereas overexpression of ARF1 did not (Figure 3E). Collectively, these results are highly indicative for a concerted role of Ysl2p, Arl1p, and Neo1p in the membrane recruitment of Gga1p and Gga2p.
Physical interaction between Ysl2p and Gga proteins
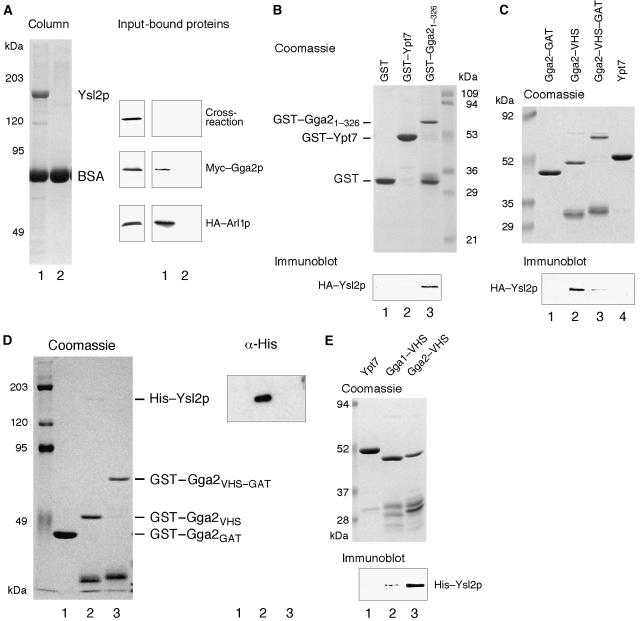
To address whether Ysl2p can bind to Gga2p, we applied several approaches. Purified His–Ysl2p was immobilized together with BSA to Sepharose 4B beads. After incubation of this affinity matrix and a control matrix containing only BSA with a yeast cell lysate containing HA–Arl1p and Myc-tagged Gga2p, both proteins were retained by the Ysl2p, but not the BSA matrix (Figure 4A). The anti-HA antibody cross-reacted with a protein present in the lysate that did not bind to either Ysl2p or BSA and thus served as a negative control (Figure 4A). A reverse binding experiment was performed using a glutathione S-transferase (GST) fusion of Gga2p including its VHS and GAT domains (GST–Gga21–326). GST alone and a GST–Ypt7p fusion served as negative controls. We detected specific binding of HA–Ysl2p to GST–Gga21–326 but no binding to the control proteins (Figure 4B). While these results confirm previous data regarding the Ysl2p–Arl1p interaction (Jochum et al, 2002), they clearly identify Gga2p as a novel binding partner of Ysl2p.
Figure 4.
Gga2p/Gga1p and Ysl2p interact in vitro. (A) Purified His–Ysl2p and BSA, coupled to CNBr-activated Sepharose 4B, were incubated with a yeast cell lysate containing HA–Arl1p and Myc–Gga2p. Here, 0.5% of the input and 10% of bound proteins were analysed by immunoblotting with anti-HA and anti-c-Myc antibodies. (B) GST–Gga21–326, GST alone, or GST–Ypt7, immobilized on glutathione Sepharose 4B, were incubated with a yeast cell lysate containing HA–Ysl2p. Bound proteins were separated by SDS–PAGE. Recombinant proteins were visualized by Coomassie staining, HA–Ysl2p by immunoblotting. The indicated immobilized GST fusions were incubated with a yeast cell lysate containing HA–Ysl2p (C) or with purified His–Ysl2p (D). Bound proteins were analysed by SDS–PAGE and immunoblotting. Recombinant proteins and His–Ysl2p were visualized by Coomassie staining, HA–Ysl2p (C) and His–Ysl2p (D) by immunoblotting. (E) The indicated immobilized GST fusions were incubated with purified His–Ysl2p. Bound proteins were analysed as described in (D).
To narrow down the domains of Gga2p involved in this interaction, GST pull downs with the individual VHS and GAT domains and HA–Ysl2p containing yeast lysates (Figure 4C) or with purified His–Ysl2p (Figure 4D), respectively, were performed. Remarkably, the individual VHS domain exhibited a clearly increased binding to Ysl2p as compared with the construct comprising the combined domains, whereas the separate GAT domain showed no binding (Figure 4C and D). As the weak interaction between the joint VHS–GAT domain and Ysl2p was only detected with yeast lysates (Figure 4C), but not with the purified protein preparation (Figure 4D), we conclude that it is mediated by another molecule.
The new finding suggests that the VHS–GAT construct adopts a conformation that cannot bind well to Ysl2p in contrast to the isolated VHS domain. Most importantly, however, our reconstitution of the Ysl2p–VHS domain interaction with purified proteins (Figure 4D) shows that it is direct. As predicted from the 70% amino-acid sequence identity within the VHS domains of Gga2p and Gga1p, Gga1p was also shown to directly interact with purified His–Ysl2p (Figure 4E). Although His–Ysl2p bound to somewhat lower extent, we can still conclude that this interaction is conserved.
Physical association of hMon2 and Gga proteins
Because the human orthologue of Ysl2p, hMon2, is a 190-kDa protein with no previously described function, we next addressed the question whether Ysl2p's key role in the binding of Gga proteins is conserved in human cells. Using a GST–VHS fusion of human Gga1 (hGga1) and HA–hMon2 present in a Δysl2 yeast lysate, indeed we detected binding to VHS hGga1 but not to Ypt7 (Figure 5A). Notably, hMon2 even interacted with yeast VHS Gga2p. Vice versa, purified His–Ysl2p bound very efficiently to human VHS hGga1 (Figure 5A). This may be due to a more favourable conformation of the human VHS fusion. In summary, the results emphasize the high conservation of this interaction across species.
Figure 5.
Conserved interaction between hMon2/His–Ysl2p and Ggas. (A) hMon2 and His–Ysl2p interact with both human and yeast VHS domains in vitro. The indicated GST fusions were incubated with a yeast cell lysate containing HA–hMon2 (lanes 1–3) or purified His–Ysl2p (lanes 4–6). Bound proteins were subjected to SDS–PAGE and visualized by Coomassie staining and immunoblotting. (B) hMon2 and hGga1/hGga3 can be co-precipitated. Extracts of non-transfected (nt) and transfected (pcDNA3-HA-GGA1, pcDNA3-HA-GGA3) HEK 293T cells were subjected to immunoprecipitation with an anti-HA antibody. Here, 2% of the input and 24% of precipitated proteins were detected by immunoblotting.
To determine whether hMon2 might physically interact with Ggas in intact cells, we transiently expressed HA-tagged versions of either hGga1 or hGga3 in HEK 293T cells and immunoprecipitated each of them from solubilized precleared supernatants using an HA antibody. Whereas endogenous hMon2 was co-precipitated with HA–hGga1 and HA–hGga3, no signal was obtained with control cell lysates (Figure 5B). Therefore, an interaction between hMon2 and Ggas can be detected in intact cells.
hMon2 mediates the subcellular localization of Ggas in human cells
Recent work has demonstrated that mammalian Gga proteins are not only involved in the sorting of cargo from the TGN but also from endosomes. hGga1 colocalizes extensively with endocytosed transferrin (Puertollano and Bonifacino, 2004) and has been implicated in retrograde trafficking of the β-secretase BACE1 from endosomes (Wahle et al, 2005). Furthermore, hGga3 associates with early endosomes, where it functions in the sorting of endocytosed cargo, in particular MPRs and EGF (Puertollano and Bonifacino, 2004).
To investigate the physiological roles of hMon2 in Gga localization, we depleted hMon2 in HeLa cells using small-interfering RNAs (siRNAs). After transfection with two RNA duplexes (siRNA nos. 2 and 3) directed against hMon2, hMon2 could be depleted to levels that are undetectable by western blotting (Figure 6A). The silencing of hMon2 was specific, because the expression levels of hGga3, γ1-adaptin (Figure 6A) or actin (not shown) were not affected by nos. 2 and 3 duplexes. Furthermore, the non-silencing (ns) control siRNA did not affect expression of any of the proteins examined (Figure 6A). In the absence of siRNA or after treatment with ns siRNA, hGga3 was concentrated in the perinuclear region and in small punctuate endomembranes (Figure 6B). After depletion of hMon2, we still observed some cellular hGga3 staining in the perinuclear region, but this pattern was superimposed by an enhanced staining in the cytoplasm and on enlarged peripheral vesicles. In the case of hGga1, after hMon2 depletion the diffuse cytoplasmic staining became more intense as compared with cells treated with ns siRNA (Supplementary Figure S1), indicating a similar redistribution of hGga1 to the cytoplasm and/or faint structures that may not be detected with the available antibody. Importantly, hMon2 depletion had no apparent effect on TGN morphology, as determined by staining against TGN46 (Figure 6B), and on Arf1 localization (Supplementary Figure S1).
Figure 6.
Depletion of hMon2 affects the localization of hGga3 and AP-1. (A) Western blots of siRNA-transfected and non-transfected cells (−) using antibodies against hMon2, γ-adaptin of AP-1, and hGga3. *cross-reacting band; ns, non-silencing siRNA. (B) HeLa cells, transfected with ns siRNA and siRNA no. 3 against hMon2, were labelled for the indicated proteins by indirect immunofluorescence. Here, 89% of cells (n=222) treated with ns siRNA showed clear perinuclear hGga3 and low cytoplasmic staining, whereas only 18% of cells (n=333) treated with siRNA no. 3 exhibited a comparable pattern. Insets show peripheral regions with enlarged vesicular structures in two-fold magnification; bar, 15 μm. (C) HeLa cells, transfected with ns siRNA and siRNA no. 3 against hMon2, were allowed to internalize surface-bound Alexa488-Tf for 10 and 30 min at 37°C, fixed, and stained for γ-adaptin of AP-1; bar, 15 μm. Data in (A–C) are representative of at least three individual experiments.
In good agreement with the observed colocalization and the proposed cooperation between Gga and AP adaptors (Dell'Angelica et al, 2000; Ghosh and Kornfeld, 2004), after hMon2 depletion we observed clear alterations in the staining patterns of both AP-1 and AP-3. In hMon2-depleted cells, γ-adaptin (Figure 6B) and the δ subunit of AP-3 (Supplementary Figure S1) were increasingly detected on pleiomorphic and enlarged structures in the cell periphery. This is in strong contrast to the compact perinuclear polarized localization of AP-1 and AP-3 in control siRNA-treated cells (Figure 6B and Supplementary Figure S1).
This observation prompted us to determine whether the enlarged peripheral foci that accumulated after hMon2 depletion correspond to endosomes. HeLa cells were allowed to internalize surface-bound Alexa488-conjugated transferrin (Alexa-Tf) and then immunostained for AP-1 whose association with the TEN is well established. This analysis provided two important clues. First, a large fraction of the enlarged peripheral AP-1 foci visible after hMon2 depletion could be loaded with Alexa-Tf internalized during a 10-min pulse, suggesting that they do correspond to early/recycling endosomes (Figure 6C). In control cells, some colocalization of AP-1 with Alexa-Tf internalized for 10 min was also apparent consistent with AP-1 residing on endosomes (Robinson, 2004), but generally observed in small foci (Figure 6C). Second, after 30 min of internalization control and hMon2-depleted cells exhibited dramatic differences in the appearance of the Alexa-Tf positive structures. Whereas in control cells Alexa-Tf was predominantly found in juxtanuclear and vesicular structures, in hMon2-depleted cells it was distributed in extended tubular structures that were discontinuously decorated with AP-1 (Figure 6C). Taken together, these data indicate that hMon2 depletion has major implications on the subcellular distribution of Ggas, concomitantly affecting the localization of AP-1 and AP-3 at endocytic sites. Moreover, the results provide first clues that trafficking of transferrin may be impaired in hMon2-depleted cells.
The localization of Gga2p to endosomal structures is also dependent on Arl1p
Given that Ysl2p has a key function in the recruitment of Gga1p and Gga2p and cooperates with Arl1p (Figure 3), the next logical step was to determine whether Arl1p is required for localization of HA–Gga2p. When compared with wild type, Δarl1 cells exhibited a more homogenous cytoplasmic HA–Gga2p staining pattern and the association with punctate structures was less obvious (Figure 7A). As the effect was less pronounced compared with Δysl2 cells, we made use of a class E vps mutant, vps27, in which expanded endosomal structures (class E compartment) accumulate. Endosomal proteins such as Ysl2p and Neo1p collapse in vps27 cells to form one or few large structures (Jochum et al, 2002; Wicky et al, 2004). A similar collapsing of Gga2p-positive structures was observed in vps27 cells (27 versus 3% in VPS27 cells; n>500), confirming previous results of an endosomal localization of Gga2p (Figure 7A; Hirst et al, 2001; Boman et al, 2002). In contrast, in the Δarl1 vps27 double mutant Gga2p was not associated with the class E compartment but still exhibited the diffuse staining pattern. These results strongly suggest that the association of Gga2p with endosomal structures is also dependent on Arl1p.
Figure 7.
Functional and biochemical links between Arl1p and Gga2p. (A) The subcellular localization of HA–Gga2p depends on Arl1p. Indirect immunofluorescence was performed with untagged (control), wild-type (wt), Δarl1, vps27, and Δarl1 vps27 cells expressing HA–Gga2p; *the expanded class E compartment. Bar, 5 μm. (B, C) Interactions between GST–Gga2 fusions and Arlp and Gga2p in vitro. Yeast cell lysates with HA–Arl1p and Myc–Gga2p were preincubated for 10 min at 30°C with either 0.5 mM GTP, GDP, or GTP-γ-S and incubated with the indicated matrix-bound GST fusions. Bound proteins were analysed as described in Figure 4B using anti-HA and anti-c-Myc antibodies.
Physical interactions between Gga2p and Arl1p and among Gga2p molecules
This finding prompted us to study the interaction between Gga2p and Arl1p using biochemical assays. Arf GTPases interact with adaptors in a GTP-dependent manner and therefore we assumed that similar conditions were relevant for an Arl1p–Gga2p interaction. A detergent-solubilized lysate obtained from a strain expressing endogenous levels of HA–Arl1p was preincubated with GTP, GDP, and the non-hydrolysable analogue GTP-γ-S under conditions to allow nucleotide exchange and then mixed with matrix-bound GST–Gga21–326 or GST–Ypt7. A weak but reproducible binding of HA–Arl1p to GST–Gga21–326 but not to GST–Ypt7 was detected in incubations containing GTP-γ-S (Figure 7B). Notably, the GTP-γ-S-loaded HA–Arl1p also interacted with VHS but not with GAT (Figure 7C), whereas the latter was previously shown to bind to yeast Arf1/2p (Zhdankina et al, 2001).
Interestingly, we also detected binding of endogenous, chromosomally expressed Myc–Gga2p to recombinant GST–Gga21–326, but only upon pretreatment of the cell lysate with GTP-γ-S (Figure 7B). Most likely, the intermolecular interaction between Gga2p molecules is dependent on a small GTPase. The identity of this protein is not Arl1p, as a similar binding between Gga2 molecules was still observed when Arl1p was absent (data not shown). Binding studies with full-length Myc–Gga2p and shorter GST–Gga2 fusions demonstrated that GAT alone did not interact, whereas VHS alone resulted in a similar interaction when compared with the combined VHS–GAT construct (Figure 7C). Thus, in this context the two domains do not act as independent modules.
In summary, we show that the Gga2p VHS domain displays a variety of novel interactions. Obviously, this complex web of interactions on Ggas that are able to form scaffolds is influenced by Gga conformation and other binding partners.
Discussion
The Ysl2p–Arl1p–Neo1p network has a vital function in the recruitment of Gga1p and Gga2p
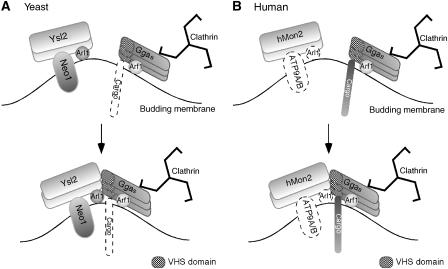
On the basis of the hypothesis that mutant Neo1-69p may induce the accumulation of unproductive intermediates that impair membrane trafficking within the endomembrane system, loss of binding partners involved might restore growth at 37°C. Such a scenario was previously suggested for Arl1p that when present in the myristoylated and GTP-bound form is detrimental in the neo1-69 background resulting in mislocalization and temperature sensitivity, whereas its loss rescues these phenotypes (Wicky et al, 2004). We predicted that deletion of other genes linked to NEO1 and ARL1 function would cause a comparable suppression. Indeed, among the tested adaptors, loss of Gga2p suppressed the temperature sensitivity of neo1-69 mutant. Moreover, Gga2p accumulated in abnormally enlarged structures in the neo1-69 strain reminiscent of those containing Arl1p and Ysl2p (Wicky et al, 2004). In perfect agreement with our hypothesis, we demonstrated that Gga2p binds directly to Ysl2p. Although loss of GGA1 did not suppress the neo1-69 mutant, likely due to the lower abundance of Gga1p, it too can directly bind to Ysl2p. Significantly, mislocalization of Ggas in the Δysl2 mutant could be specifically rescued by overexpression of Arl1p, but not Arf1p, corroborating the role of Arl1p in this process. The fact that additional Neo1p also suppressed Gga2p mislocalization in Δysl2 cells is further compelling genetic evidence for the concept of cooperation between Ysl2p, Arl1p, and Neo1p during Gga recruitment (Figure 8A). Biochemical evidence for molecular links between Gga2p and Ysl2p as well as Arl1p is manifold. Gga2p was retained on a Ysl2p microaffinity column, similar to Arl1p. Vice versa, GST fusions of Gga1/2p revealed interactions with both Ysl2p and activated Arl1p, suggesting that Gga2p is a novel effector of GTP–Arl1p. Using purified proteins, the Ysl2p–Gga1/2p interaction was shown to be direct, mediated by the VHS domain. Whether the Gga2p–Arl1p interaction is direct is not known yet, but given that loss of Arl1p did affect the subcellular targeting of Gga2p (Figure 7A) and Arl1p overexpression rescued the defective recruitment when Ysl2p is missing (Figure 3E), this is quite likely. Finally, our results concerning the inter-Gga2p-binding ability in dependence of GTP-γ-S further corroborate the idea that Ggas can form multimers on membranes. Taken together, the newly identified interactions derived from this work are schematically illustrated in Figure 8A.
Figure 8.
Comparison of network interactions between Ysl2p/hMon2, Arl1p, Neo1p orthologues, and Gga clathrin adaptors in yeast and humans. (A) Multimeric Ysl2p, small GTPase Arl1p, and Neo1p cooperate during vesicle formation and endosome biogenesis. All direct contacts indicated in the cartoon represent experimentally supported physical interactions. Ggas can bind to clathrin and are recruited to membranes by GTP–Arf1p. Significantly, Arf1p interaction appears not to be sufficient for Gga function or localization and cargo (indicated by a dotted symbol) directly binding to Ggas through VHS has not yet been identified. Our results show that Ysl2p directly interacts with Gga1p/Gga2p through VHS; GTP–Arl1p binds to Gga2p; Neo1p is linked to Gga recruitment by virtue of a mutant allele, neo1-69, and by suppression of Δysl2; membrane-bound Gga2p interacts with itself. (B) Human homologues of the Ysl2p–Arl1p–Neo1p complex exist, but biochemical and functional links among them are unknown. Arl1 and the putative human Neo1p orthologues, ATP9A/B, are represented by dotted symbols. Cargo that directly binds to Ggas through VHS has been identified. GTP–Arf1 has a central role in the membrane docking of Ggas through GAT. Binding of Ggas to clathrin occurs as described in (A). Our new data show that hMon2 interacts with Ggas in vivo and in vitro (through VHS), and loss of hMon2 has an impact on Gga targeting.
hMon2, the human orthologue of Ysl2p, represents a novel Gga VHS-binding partner
In human cells, the dramatic effect of hMon2 depletion on localization of hGga1 and hGga3 is fully consistent with the findings in yeast. Further, independent biochemical evidence for a physiologically relevant interaction between Gga proteins and hMon2 was provided by the co-isolation of hMon2 upon precipitation of hGga1 and hGga3, respectively. Complementary in vitro binding studies nicely confirmed our prediction that hMon2 interacts with Ggas through their VHS domain. Thus, this study has highlighted a previously unknown component with a central role in Gga recognition and targeting, the human orthologue of Ysl2p (Figure 8B). Accordingly, we then predict that human orthologues of yeast Arl1p and Neo1p would co-function with hMon2 in analogy to the yeast proteins (Figure 8B).
Similar to Ysl2p/hMon2, human GBF1 was recently shown to colocalize and directly interact with Ggas (Lefrançois and McCormick, 2007). Although details of this interaction are still unknown, this resemblance is striking given the extensive homology shared by the large Sec7 GEFs of the GBF/BIG families and the Mon2 family (Ramaen et al, 2007). As GBF1 localizes to the cis-side of the Golgi, it is fully consistent that homologues such as Ysl2p/hMon2 are involved in Gga recruitment at other cellular sites. A remarkable difference between the GBF and Mon2 families is, however, their primary cooperation with either Arf1 or Arl1, respectively.
Role of Ysl2p/hMon2 in the process of Gga-mediated coat recruitment
Until now, the concept of Arf being the critical component in the process of Gga recruitment to membranes has dominated. In mammalian cells, GTP–Arf recruits Gga proteins to the TGN/TEN membrane by interacting with the GAT domain (Figure 8B; Boman et al, 2000; Dell'Angelica et al, 2000; Puertollano et al, 2001). In yeast, however, Ggas do not require Arf for their localization and function, although binding to Arf has been demonstrated (Figure 8A; Boman et al, 2002).
Our findings establish that Ysl2p/hMon2 contributes significantly to this process of Gga recognition and targeting and that this role is highly conserved from yeast to humans (Figure 8A and B). The immunofluorescence analysis clearly suggested that the cytoplasmic pool of hGga1 and hGga3 increased after hMon2 depletion. However, independent evidence for a possible release of Ggas into the cytosol could not be obtained. Consistent with other reports, after subcellular fractionation of lysed cells the majority of Gga proteins (80% of hGga3; A-M Bürger and B Singer-Krüger, unpublished results) can be already detected in the soluble fraction, likely due to low-affinity interactions with binding partners on the membrane. In addition to the enhanced diffuse cytoplasmic staining, in hMon2-depleted cells we noticed the localization of a fraction of hGga3 on enlarged peripheral structures. Besides loosing a critical Gga recruitment factor, the depletion of hMon2 may affect other cellular functions that could be independent of Gga targeting. Given the strong conservation of Ysl2p and hMon2, we must assume roles for hMon2 in membrane trafficking within the endomembrane system similar to those already demonstrated for Ysl2p (Jochum et al, 2002; Wicky et al, 2004). Thus, the enlarged hGga3-positive structures might represent transport intermediates building up upon loss of hMon2. They may still include cargo with signals recognized by Ggas. As a subset of the enlarged hGga3-positive structures were also shown to contain transferrin (A-M Bürger and B Singer-Krüger, unpublished results), they are derived from endosomal trafficking. The concomitant redistribution of AP-1 and AP-3 to the enlarged peripheral structures after hMon2 silencing could be caused by impaired membrane trafficking as well as by the proposed collaborative roles of Ggas and APs. The combined effect on Ggas and APs is also coherent with our genetic data that loss of the AP-1/AP-3 β subunits Apl2p and Apl6p caused a weak suppression of the neo1-69 growth defect (Figure 1A). Thus, intricate links of the monomeric and tetrameric adaptors are evident both in mammals and yeast (Costaguta et al, 2001; Ghosh and Kornfeld, 2004). Further evidence for a related function of Ysl2p and its associated network and hMon2 stems from the morphological alterations of the transferrin-containing structures observed after hMon2 depletion (Figure 6C). This phenotype may be caused by defects in membrane scission events at the endocytic recycling compartment. It strongly reminds on previously described alterations of transferrin-positive structures in HeLa cells depleted of AAK1, a kinase and putative regulator of AP-1 (Henderson and Conner, 2007), and on the above-described tubular membrane protrusions displayed by neo1 mutants with a characteristic increase in surface to volume ratio (Wicky et al, 2004). Clearly, future studies will focus on resolving the molecular details of hMon2 localization and function within the TEN.
As we have shown, the Ysl2p/hMon2–Gga binding is mediated by the VHS domain, which in mammalian cells is considered to mediate binding to an acidic cluster dileucine motif, DXXLL, present within the cytoplasmic tails of cargo such as MPRs and sortilin (Bonifacino, 2004; Figure 8B). In yeast, such a direct cargo-binding role of Gga has not been discovered (Figure 8A). As the residues within the mammalian VHS domain that bind to DXXLL signals are not conserved in yeast VHS domains, one interpretation has been that they recognize different motifs within putative cargo. Notably, hGga1 VHS binds to both Ysl2p and hMon2, and the reverse is true for yeast Gga2p VHS. The question whether this interaction involves a short linear peptide motif is still unknown. It will also be important to determine whether VHS can bind to hMon2 and cargo simultaneously or in a defined order. Indeed, the question to what extent cargo actively helps to recruit coats onto the donor membrane has been a matter of debate. Because in yeast no VHS-binding cargo has been identified so far, the possibility that at least in this organism cargo may not be involved during Gga-mediated coat recruitment and, generally, that coat recruitment and cargo selection may be independent and successive steps has to be further considered, even though in the mammalian system moderate overexpression of DXXLL-containing cargo has be found to enhance membrane association of Ggas (Hirst et al, 2007). Most likely, proteins such as Ysl2p/hMon2 represent fundamental docking scaffolds in a selective Gga recruitment cascade thereby linking the adaptors directly to cargo at specific subcellular sites when VHS-binding signals have evolved. As shown in this and previous studies, Ysl2p and homologous proteins appear to cooperate with members of the Drs2 subfamily of P-type ATPases thought to act as lipid translocases. The potential role of flippase-catalysed phospholipid translocation in the generation of curved membranes during budding has been discussed for many years (see review by Graham, 2004). Flippases likely contribute to vesicle formation, for example by producing positive curvature and priming the membrane for budding or by directly working together with the vesicle budding machinery to generate positive curvature and stimulate the assembly of coat components. Supportive evidence for this concept are the physical interactions revealed between Drs2p/Neo1p and Gea2p/Ysl2p (Chantalat et al, 2004; Wicky et al, 2004) and their links to clathrin and Ggas, respectively. Flippases may also concentrate certain phospholipids on the cytoplasmic leaflet stimulating the binding of effector molecules implicated in bud formation. Last but not the least, conformational changes linked to ATP hydrolysis might regulate protein interactions and thereby favour budding reactions. Consistent with this possibility is that expression of mutant Neo1-69p causes enhanced recruitment of elements of the budding machinery such as Ysl2p, Arl1p, and Gga2p and results in the described morphological defects. Although much remains to be learned about the exact interplay among Ysl2p/hMon2, Arf/Arl small GTPases, flippases, cargo, and Ggas and the order in which they function, our results provide a foundation to understand the conserved contribution of hMon2/Ysl2p to the process of Gga-dependent trafficking within the endomembrane system.
Additional roles of Arl1p in vesicle formation at donor membranes
The revelation of physical and functional links between Gga2p and Arl1p prompts us to propose a further role of Arl1p at sites where budding intermediates are generated (Figure 8). The major function ascribed so far to Arl1p is its role in the recruitment of GRIP domain golgins (Lu and Hong, 2003; Panic et al, 2003; Setty et al, 2003). These dimeric proteins are thought to act as vesicle tethers that capture transport carriers destined to deliver cargo to an acceptor compartment. One attractive possibility is that Arf1p and Arl1p cooperate during the assembly of the budding machinery to provide additional specificity, to couple different steps, and to allow cross-talk to downstream components and signalling pathways ensuring directionality and continuity. Examples of GTPase cascades involving members of one and the same subfamily (such as Rab and Arl cascades) or different subfamilies linked directly or through the action of accessory proteins such as GEFs have been established (Munro, 2005; Grosshans et al, 2006). For the Arf/Arl proteins, a similar cascade concept could provide clues to explain the multitude of Arf functions. The growing identification of common Arf/Arl effectors and regulators (van Valkenburgh et al, 2001; Lu and Hong, 2003; Bowzard et al, 2007) corroborates a model of cooperation of Arf/Arl small GTPases. During coat recruitment, the potential significance of Arl1p/Ysl2p engaging the Gga VHS domain and Arf1p binding the GAT domain could, for example, be to help to induce and/or stabilize (through regulatory factors) the open, membrane-bound conformation of Ggas thereby favouring downstream interactions with clathrin, APs and other accessory proteins through the C-terminal domains of Ggas. The challenge will now be to further elucidate the exact order and regulation of the dynamic and multiple interactions between Ggas and binding partners during vesicular transport.
Materials and methods
Strains and media
Yeast strains (Table I) were grown at 25°C unless otherwise indicated in standard media as described (Wicky et al, 2004). Integration cassettes were generated by PCR using pFA6a-kanMX6, pFA6a-3HA-kanMX6, and pFA6a-13Myc-kanMX6 and inserted at the chromosomal GGA1, GGA2, APL2, and APL6 loci in the diploid strain RH1201 by homologous recombination. Sequences of oligonucleotides used to generate the integration cassettes are available upon request. HeLa and HEK 293T cells were grown at standard conditions in DMEM with 10% FBS.
Table 1.
Strains used by Singer-Krüger et al
| Yeast strain | Genotype | Source |
|---|---|---|
| BS64 | MATa his4 ura3 leu2 lys2 bar1-1 | Wicky et al (2004) |
| BS695 | MATα his4 ura3 leu2 lys2 ysl2∷kanr bar1-1 | This study |
| BS917 | MATa his4 ura3 leu2 lys2 neo1∷kanr bar1-1+pRS315-neo1-69 | Wicky et al (2004) |
| BS1121 | MATα ura3 leu2 lys2 YSL2∷3-HA-HIS5 (S. pombe) bar1-1 | Wicky et al (2004) |
| BS1283 | MATa ura3 leu2 lys2 neo1∷kanr ARL1∷3-HA-HIS5 (S. pombe) bar1-1+pRS315-neo1-69 | Wicky et al (2004) |
| BS1323 | MATα ura3 leu2 lys2 neo1∷kanr arl1∷kanr bar1-1+pRS315-neo1-69 | Wicky et al (2004) |
| BS1347 | MATa/α his3Δ1/ his3Δ1 ura3-52/ura3-52 leu2/leu2 trp1-289/trp1-289 | Invitrogen |
| BS1722 | MATα ura3 leu2 lys2 his4 GGA1∷3-HA-kanr bar1-1 | This study |
| BS1779 | MATa his4 ura3 leu2 lys2 GGA2∷3-HA-kanr neo1∷kanr bar1-1+pRS316-NEO1 | This study |
| BS1787 | MATa ura3 leu2 lys2 his4 GGA1∷3-HA-kanr ysl2∷kanr bar1-1 | This study |
| RH1201 | MATa/α his4/his4 ura3/ura3 leu2/leu2 lys2/lys2 bar1-1/bar1-1 | H Riezman, Geneva |
| YML278 | MATα his4 ura3 leu2 lys2 apl6∷kanr neo1∷kanr bar1-1+pRS315-neo1-69 | This study |
| YML281 | MATa his4 ura3 leu2 lys2 gga2∷kanr neo1∷kanr bar1-1+pRS315-neo1-69 | This study |
| YML290 | MATα his4 ura3 leu2 lys2 gga1∷kanr neo1∷kanr bar1-1+pRS315-neo1-69 | This study |
| YML296 | MATα his4 ura3 leu2 lys2 apl2∷kanr neo1∷kanr bar1-1+pRS315-neo1-69 | This study |
| YML303 | MATα his4 ura3 leu2 lys2 GGA2∷3-HA-kanr bar1-1 | This study |
| YML307 | MATa his4 ura3 leu2 lys2 GGA2∷3-HA-kanr neo1∷kanr bar1-1+pRS315-neo1-69 | This study |
| YML333 | MATa ura3 leu2 lys2 vps27 GGA2∷3-HA-kanr | This study |
| YML341 | MATα his4 ura3 leu2 lys2 arl3∷kanr neo1∷kanr bar1-1+pRS315-neo1-69 | This study |
| YML360 | MATa his4 ura3 leu2 lys2 GGA2∷3-HA-kanr arl1∷URA3 bar1-1 | This study |
| YML363 | MATα his4 ura3 leu2 lys2 GGA2∷3-HA-kanr ysl2∷kanr bar1-1 | This study |
| YML371 | MATα his4 ura3 leu2 lys2 ypt51∷LYS2 neo1∷kanr bar1-1+pRS315-neo1-69 | This study |
| YML376 | MATα his4 ura3 leu2 ypt7∷URA3 neo1∷kanr bar1-1+pRS315-neo1-69 | This study |
| YML397 | MATα ura3 leu2 lys2 vps27 GGA2∷3-HA-kanr arl1∷URA3 | This study |
| YML407 | MATα ura3 leu2 lys2 GGA2∷13-Myc-kanr ARL1∷3-HA-HIS5 (S. pombe) bar1-1 | This study |
| YML413 | MATα his4 ura3 leu2 lys2 arf1∷kanr neo1∷kanr bar1-1+pRS315-neo1-69 | This study |
Plasmids
pYSL2-YES2/CT was generated by PCR, using pBS207 (Jochum et al, 2002) and pYES2/CT (Invitrogen) as starting material. hMon2 was cloned by PCR into pRS425-ADH-3xHA using DKFZp686F0546Q (RZPD, Berlin) as starting material. Plasmids pRS426-ARL1, pFL-ARF1, pRS315-NEO1, and pmycTLG1 have been described (Jochum et al, 2002; Wicky et al, 2004). The pGEX4-Gga21–326 plasmid was constructed by inserting a PCR fragment encoding aa 1–326 of Gga2p into the bacterial expression vector pGEX-4T-1 (Amersham). Likewise, pGEX4-Gga2VHS (aa 1–180 of Gga2p), pGEX4-Gga2GAT (aa 182–326), pGEX4-Gga1VHS (aa 1–176 of Gga1p), and pGEX4-hGga1VHS (aa 1–171 of hGga1) were constructed. All constructs were confirmed by sequencing. The plasmids pGEX5-Ypt7 (Jochum et al, 2002; Böttcher et al, 2006), pcDNA3-HA-GGA1, and pcDNA3-HA-GGA3 (Boman et al, 2000) have been described.
Indirect immunofluorescence of yeast cells
Indirect immunofluorescence microscopy was performed as described (Wicky et al, 2004).
Purification of His–Ysl2p, coupling to CNBr-activated Sepharose and affinity chromatography
Yeast cells expressing His–Ysl2p from pYSL2/CT were resuspended in 20 mM Hepes/NaOH pH 7.8, 150 mM NaCl buffer with protease inhibitors and lysed in a French press. The precleared lysate was subjected to IMAC using Ni Sepharose high performance (Amersham). His–Ysl2p was eluted with an imidazole gradient and dialysed against 20 mM Hepes/NaOH pH 7.8, 1 mM MgCl2, 1 mM DTT, 50 mM NaCl, 10% (w/v) glycerol. Approximately 30 μg of purified His–Ysl2p were coupled to CNBr-activated Sepharose. BSA was coupled to the control beads. At 1 h after the initial addition of either His–Ysl2p or BSA, another 70 μg of BSA were added to both types of matrices. Coupling was completed according to standard procedures. Binding assays were performed with 1 ml of yeast cell lysates as described for GST pull downs.
Affinity chromatography with GST fusion proteins
GST pull downs with yeast cell lysates were performed as described (Böttcher et al, 2006). To detect binding of HA–Arl1p and c-Myc–Gga2p, cell lysates were preincubated with either 0.5 mM GDP, GTP, or GTPγS for 10 min at 30°C before binding to matrix-bound GST fusions. Binding assays with purified His–Ysl2p (10 μg) contained 10 μg GST fusion protein.
RNA interference and indirect immunofluorescence of HeLa cells
Two siRNA duplexes against human hMon2, no. 2 (5′-CAGAGAUAUUAUAGAACAA-3′) and no. 3 (5′-AGAACUCAAUAUUAGUUUA-3′), and a ns siRNA duplex (AllStars Negative Control siRNA) were from Qiagen. HeLa cells were transfected using the HiPerFect transfection reagent (Qiagen). For efficient knockdown, two transfections were performed 3 days apart, and experiments were carried out 2 days after the second knockdown. Cell extracts were prepared in 20 mM Hepes/KOH, pH 7.4, 1 mM EDTA, 0.1 M KCl, 1% TX100, including protease inhibitors. For indirect immunofluorescence, HeLa cells were fixed with 4% paraformaldehyde, followed by permeabilization with 0.2% Tween 20. Primary antibodies were mouse anti-hGga3 (BD Transduction Laboratories; 1:100), rabbit anti-hGga1 (M Robinson, Cambridge, 1:1000), sheep anti-TGN46 (Serotec; 1:200), mouse anti-γ-adaptin of AP-1 (Sigma; 1:300), mouse anti-δ of AP-3 (Developmental Studies Hybridoma Bank; 1:400), and mouse anti-Arf1 (Affinity Bioreagents; 1:100). Secondary antibodies were goat anti-mouse IgG-Alexa488, goat anti-mouse IgG-Alexa546, goat anti-rabbit IgG-Alexa488, and donkey anti-sheep/goat IgG-FITC. Internalization assays of cells transfected with siRNA were performed as described by Puertollano and Bonifacino (2004). Alexa488-Tf was bound to precooled cells for 30 min at 4°C. After washing, cells were incubated at 37°C for 10 and 30 min. Images were obtained from projections of z-stack series (0.5 μm intervals) using a Leica confocal laser scanning microscope (TCS SP2 and TCS SL) and Leica Confocal Software. Fluorescence intensities were analysed using ImageJ software.
Immunoprecipitation with HEK 293T cell lysates
HEK 293T cells were transfected with pcDNA3-HA-GGA1 and pcDNA3-HA-GGA3 using Lipofectamin2000 (Invitrogen) 18 h before lysis in ice-cold buffer (20 mM Hepes/KOH, pH 7.4, 1 mM EDTA, 115 mM KCl, 5 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.8% NP40, protease inhibitors). For immunoprecipitation, cleared lysates were incubated 2 h at 4°C with rat monoclonal anti-HA antibody (3F10; Roche). Immunocomplexes were bound to Protein A Sepharose 4B beads. After extensive washing, bound proteins were released with SDS–PAGE sample buffer and analysed by immunoblotting.
Generation of a polyclonal antibody against hMon2
A synthetic peptide representing the internal sequence DVLHRYIEDERLSGKC was coupled to keyhole limpet haemocyanin. The immunization of rabbits was carried out by Charles River Laboratories (Germany).
Supplementary Material
Supplementary Figure S1
Supplementary Figure Legends
Acknowledgments
We are grateful to B Bowzard, J Holthuis, R Kahn, H Riezman, and M Robinson for kindly providing plasmids, strains, and antibodies. We thank S Nußberger for access to his cell culture facility and O Schlicker for support with the confocal microscopy. We acknowledge H Rudolph and S Barbosa for critical reading of the manuscript and helpful discussions. This work was supported by a grant from the DFG to BS-K (Si 635).
References
- Boman A, Zhang C, Zhu X, Kahn RA (2000) A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol Biol Cell 11: 1241–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman AL, Salo PD, Hauglund MJ, Strand NL, Rensink SJ, Zhdankina O (2002) ADP-ribosylation factor (ARF) interaction is not sufficient for yeast GGA protein function or localization. Mol Biol Cell 13: 3078–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS (2004) The GGA proteins: adaptors on the move. Nat Rev Mol Cell Biol 5: 23–32 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Rojas R (2006) Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol 7: 568–579 [DOI] [PubMed] [Google Scholar]
- Böttcher C, Wicky S, Schwarz H, Singer-Krüger B (2006) Sjl2p is specifically involved in early steps of endocytosis intimately linked to actin dynamics via the Ark1p/Prk1p kinases. FEBS Lett 580: 633–641 [DOI] [PubMed] [Google Scholar]
- Bowzard JB, Cheng D, Peng J, Kahn RA (2007) ELMOD2 is an Arl2 GTPase-activating protein that also acts on Arfs. J Biol Chem 282: 17568–17580 [DOI] [PubMed] [Google Scholar]
- Chantalat S, Park SK, Hua Z, Gobin R, Peyroche A, Rambourg A, Graham TR, Jackson CL (2004) The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J Cell Sci 117: 711–722 [DOI] [PubMed] [Google Scholar]
- Costaguta G, Stefan CJ, Bensen ES, Emr SD, Payne GS (2001) Yeast Gga coat proteins function with clathrin in Golgi to endosome transport. Mol Biol Cell 12: 1885–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC, Puertollano R, Mullins C, Aguilar RC, Vargas JD, Hartnell LM, Bonifacino JS (2000) GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J Cell Biol 149: 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efe JA, Plattner F, Hulo N, Kressler D, Emr SD, Deloche O (2005) Yeast Mon2p is a highly conserved protein that functions in the cytoplasm-to-vacuole transport pathway and is required for Golgi homeostasis. J Cell Sci 118: 4751–4764 [DOI] [PubMed] [Google Scholar]
- Ghosh P, Kornfeld S (2004) The GGA proteins: key players in protein sorting at the trans-Golgi network. Eur J Cell Biol 83: 257–262 [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Whyte JRC, Panic B, Munro S (2006) Mon2, a relative of large Arf exchange factors, recruits Dop1 to the Golgi apparatus. J Biol Chem 281: 2273–2280 [DOI] [PubMed] [Google Scholar]
- Graham TR (2004) Flippases and vesicle-mediated protein transport. Trends Cell Biol 14: 670–677 [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P (2006) Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103: 11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DM, Conner SD (2007) A novel AAK1 splice variant functions at multiple steps of the endocytic pathway. Mol Biol Cell 18: 2698–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Lindsay MR, Robinson MS (2001) GGAs: roles of the different domains and comparison with AP-1 and clathrin. Mol Biol Cell 12: 3573–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Seaman MNJ, Buschow SI, Robinson MS (2007) The role of cargo proteins in GGA recruitment. Traffic 8: 594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z, Graham TR (2003) Requirement for Neo1p in retrograde transport from the Golgi complex to the endoplasmic reticulum. Mol Biol Cell 14: 4971–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum A, Jackson D, Schwarz H, Pipkorn R, Singer-Krüger B (2002) Yeast Ysl2p, homologous to Sec7 domain guanine nucleotide exchange factors, functions in endocytosis and maintenance of vacuole integrity and interacts with the Arf-like small GTPase Arl1p. Mol Cell Biol 22: 4914–4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA (1995) The Arf Subfamily. Oxford, UK: Oxford University Press [Google Scholar]
- Lefrançois S, McCormick PJ (2007) The Arf GEF GBF1 is required for GGA recruitment to Golgi membranes. Traffic 8: 1440–1451 [DOI] [PubMed] [Google Scholar]
- Lu L, Hong W (2003) Interaction of Arl1–GTP with GRIP domains recruits autoantigens Golgin-97 and Golgin-245/p230 onto the Golgi. Mol Biol Cell 14: 3767–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S (2005) The Arf-like GTPase Arl1 and its role in membrane traffic. Biochem Soc Trans 33: 601–605 [DOI] [PubMed] [Google Scholar]
- Panic B, Whyte JR, Munro S (2003) The ARF-like GTPases Arl1p and Arl3p act in a pathway that interacts with vesicle-tethering factors at the Golgi apparatus. Curr Biol 13: 405–410 [DOI] [PubMed] [Google Scholar]
- Pasqualato S, Renault L, Cherfils J (2002) Arf, Arl, Arp and Sar proteins: a family of GTP-binding proteins with a structural device for ‘front–back' communication. EMBO Rep 3: 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R, Bonifacino JS (2004) Interactions of GGA3 with the ubiquitin sorting machinery. Nat Cell Biol 6: 244–251 [DOI] [PubMed] [Google Scholar]
- Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS (2001) The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell 105: 93–102 [DOI] [PubMed] [Google Scholar]
- Ramaen O, Joubert A, Simister P, Belgareh-Touze N, Olivares-Sanchez MC, Zeeh J-C, Chantalat S, Golinelli-Cohen M-P, Jackson CL, Biou V, Cherfils J (2007) Interactions between conserved domains within homodimers in the Big1, Big2 and Gbf1 Arf guanine nucleotide exchange factors. J Biol Chem 282: 28834–28842 [DOI] [PubMed] [Google Scholar]
- Robinson MS (2004) Adaptable adaptors for coated vesicles. Trends Cell Biol 14: 167–174 [DOI] [PubMed] [Google Scholar]
- Rosenwald AG, Rhodes MA, Van Valkenburgh H, Palanivel V, Chapman G, Boman A, Zhang CJ, Kahn RA (2002) ARL1 and membrane traffic in Saccharomyces cerevisiae. Yeast 19: 1039–1056 [DOI] [PubMed] [Google Scholar]
- Setty SR, Shin ME, Yoshino A, Marks MS, Burd CG (2003) Golgi recruitment of GRIP domain proteins by Arf-like GTPase 1 is regulated by Arf-like GTPase 3. Curr Biol 13: 401–404 [DOI] [PubMed] [Google Scholar]
- Van Valkenburgh H, Shern JF, Sharer JD, Zhu X, Kahn RA (2001) ADP-ribosylation factors (ARFs) and ARF-like 1 (ARL1) have both specific and shared effectors. J Biol Chem 276: 22826–22837 [DOI] [PubMed] [Google Scholar]
- Wahle T, Prager K, Raffler N, Haass C, Famulok M, Walter J (2005) GGA proteins regulate retrograde transport of BACE1 from endosomes to the trans-Golgi network. Mol Cell Neurosci 29: 453–461 [DOI] [PubMed] [Google Scholar]
- Wicky S, Schwarz H, Singer-Krüger B (2004) Molecular interactions of yeast Neo1p, an essential member of the Drs2 family of aminophospholipid translocases, and its role in membrane trafficking within the endomembrane system. Mol Cell Biol 24: 7402–7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdankina O, Strand NL, Redmond JM, Boman AL (2001) Yeast GGA proteins interact with GTP-bound Arf and facilitate transport through the Golgi. Yeast 18: 1–18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure Legends