Abstract
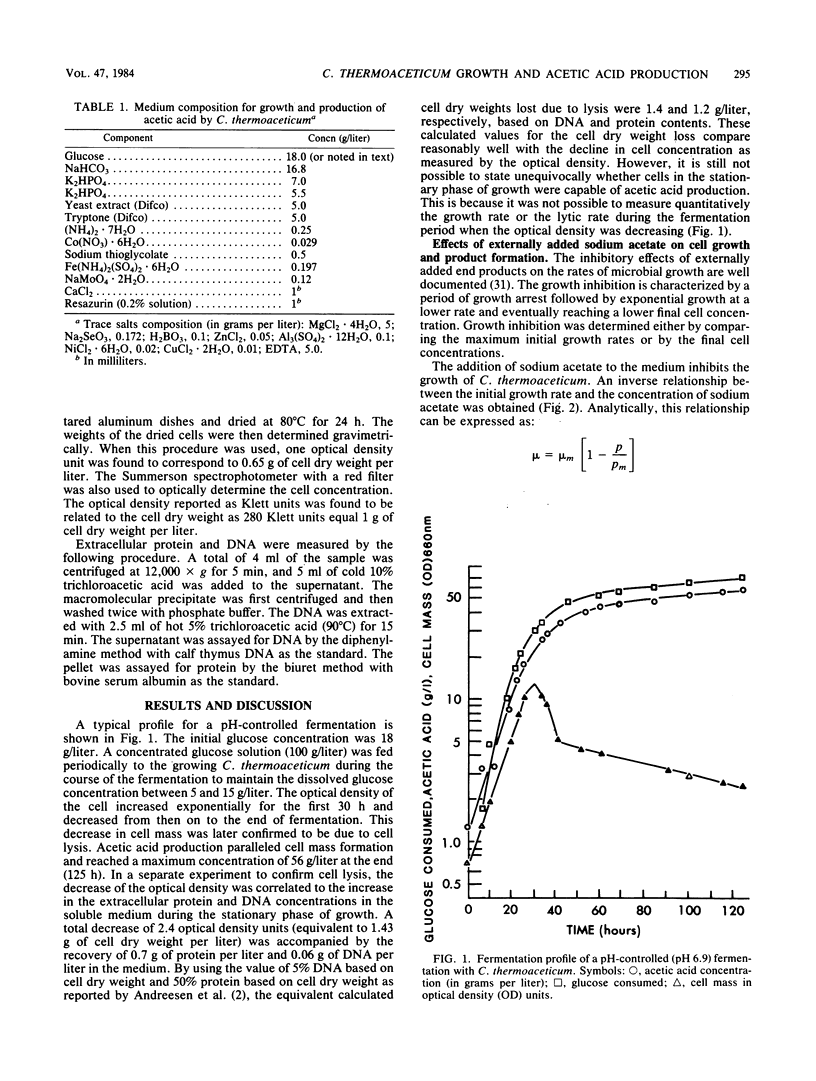
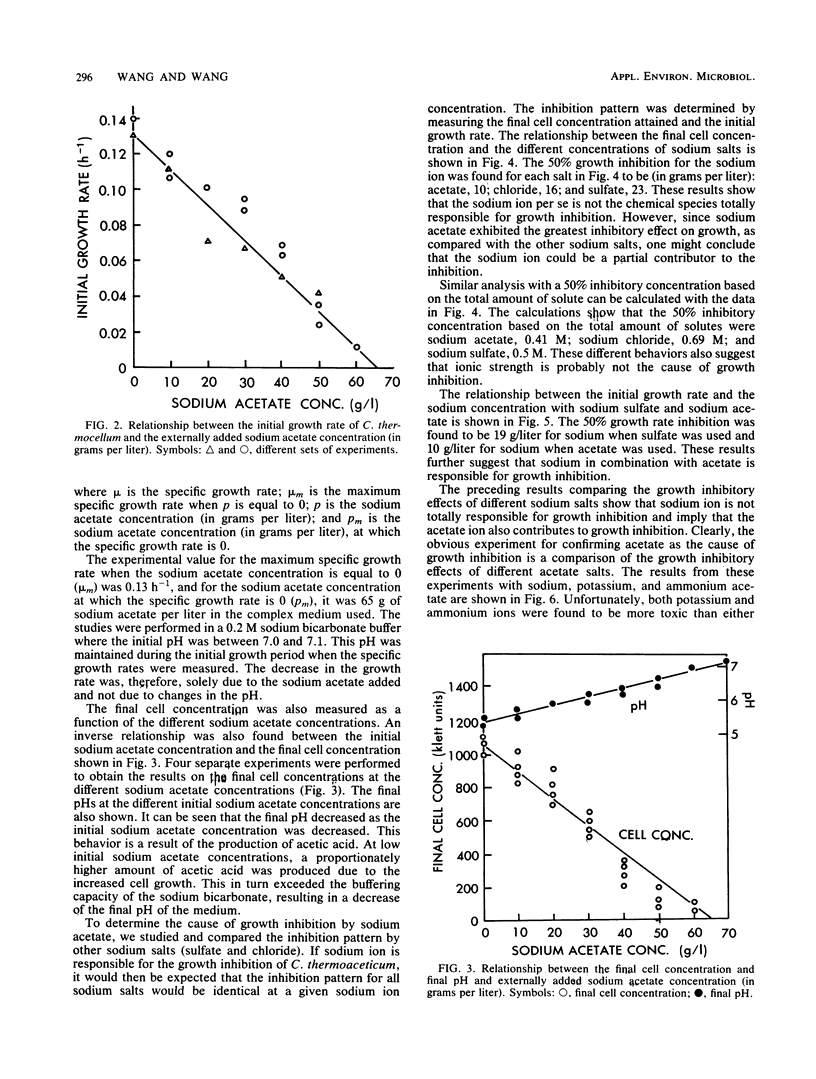
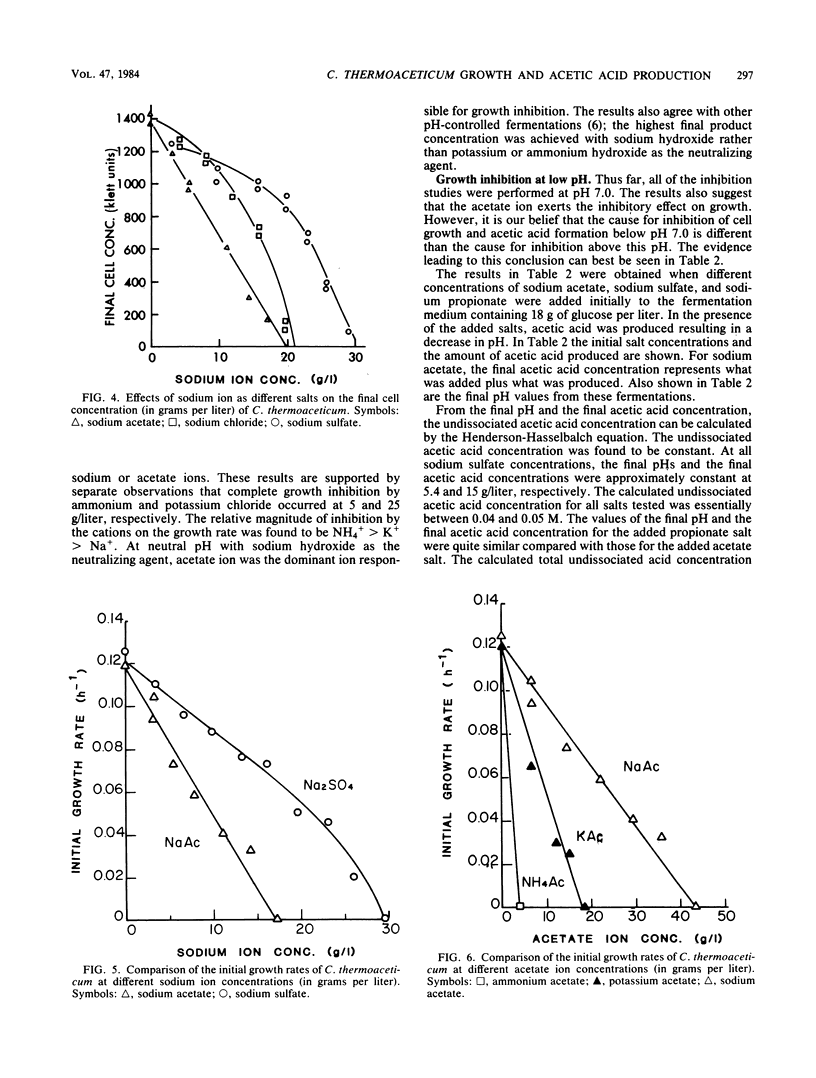
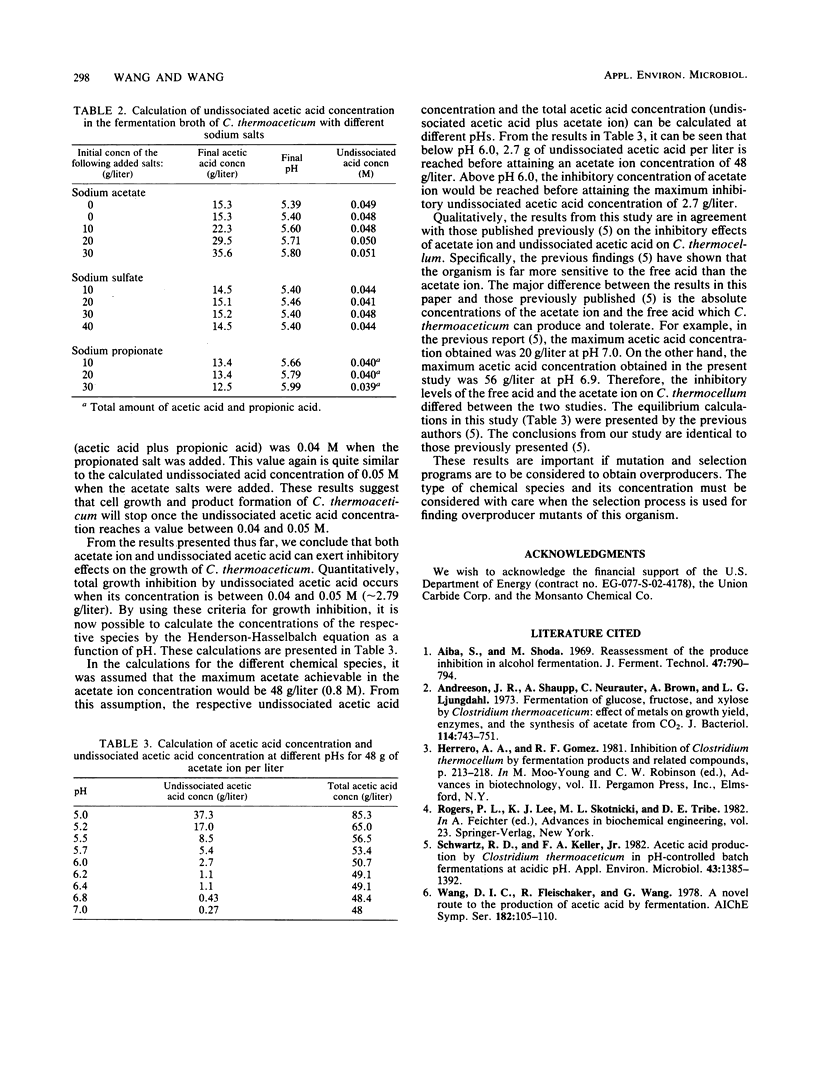
The production of acetic acid by Clostridium thermoaceticum was studied by using batch fermentations. In a pH-controlled fermentation with sodium hydroxide (pH 6.9), this organism was able to produce 56 g of acetic acid per liter. On the other hand, when the pH was not controlled and was decreased during fermentation to 5.4, the maximum attainable acetic acid concentration was only 15.3 g/liter. To obtain a better understanding of the end product inhibition, various salts were tested to determine their effect on the growth rate of C. thermoaceticum. An inverse linear relationship between the growth rate and the final cell concentration to the sodium acetate concentration was found. By using different concentrations of externally added sodium salts, the relative growth inhibition caused by the anion was found to be in the order of acetate > chloride > sulfate. Various externally added cations of acetate were also examined with respect to their inhibitory effects on growth. The relative magnitude of inhibition on the growth rate was found to be ammonium > potassium > sodium. The combined results have shown that the undissociated acetic acid was much more inhibitory than the ionized acetate ion. Complete growth inhibition resulted when the undissociated acetic acid concentration was between 0.04 and 0.05 M and when the ionized acetate concentration was 0.8 M. Therefore, at low pH (below 6.0), undissociated acetic acid is responsible for growth inhibition, and at high pH (above 6.0), ionized acetate ion is responsible for growth inhibition.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. D., Keller F. A. Acetic Acid Production by Clostridium thermoaceticum in pH-Controlled Batch Fermentations at Acidic pH. Appl Environ Microbiol. 1982 Jun;43(6):1385–1392. doi: 10.1128/aem.43.6.1385-1392.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


