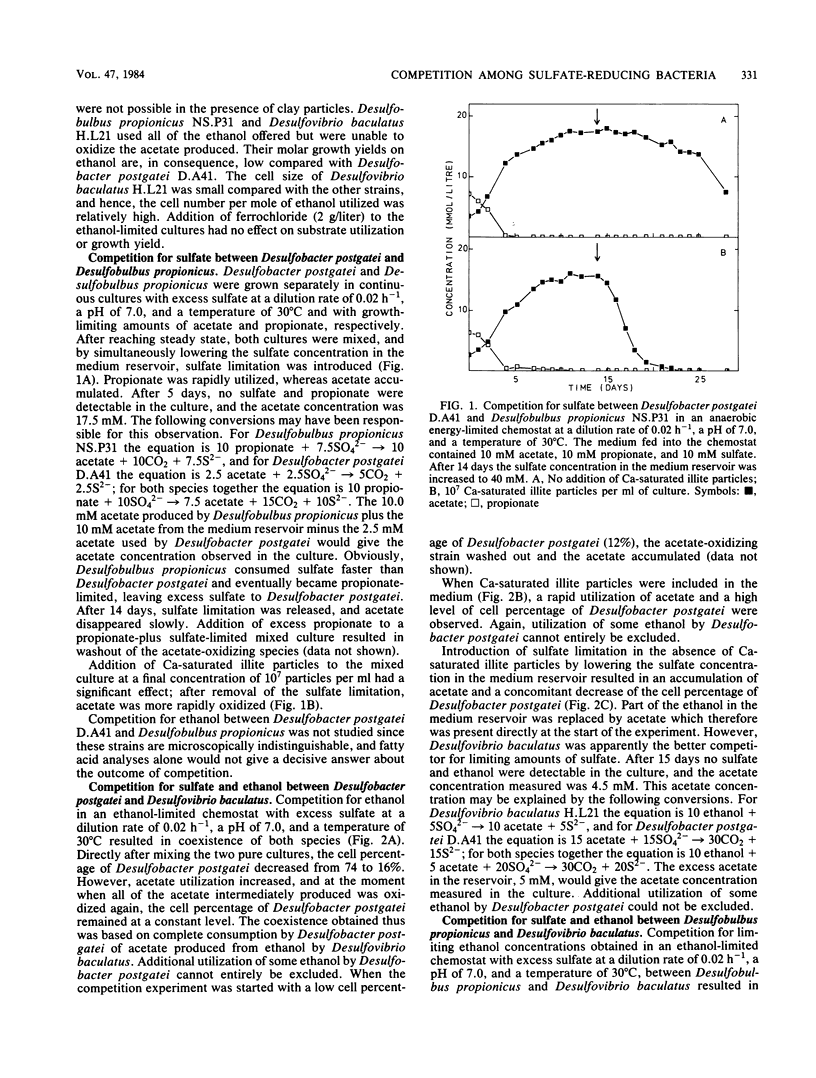
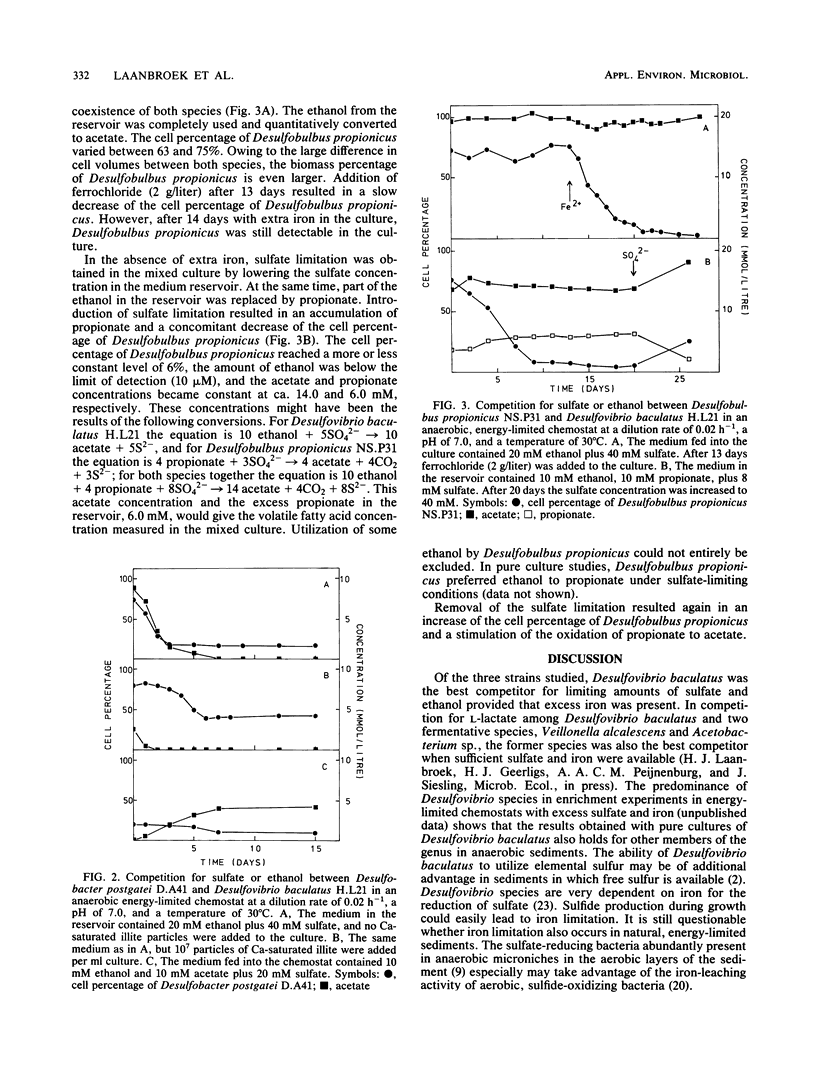
Abstract
Competition for sulfate and ethanol among Desulfobacter, Desulfobulbus, and Desulfovibrio species isolated from estuarine sediments was studied in energy-limited chemostats. Desulfovibrio baculatus was the most successful competitor for limiting amounts of sulfate and ethanol, followed by Desulfobulbus propionicus. The success of Desulfovibrio baculatus was dependent on the availability of sufficient iron. Of the three species studied, Desulfobacter postgatei was the least successful competitor for limiting amounts of sulfate. Although stimulating the growth of Desulfobacter postgatei, addition of Ca-saturated illite particles to culture media did not affect the outcome of competition for sulfate. Thus, under sulfate limitation acetate accumulated. This phenomenon was briefly discussed in relation to the flow of electrons during anaerobic mineralization in marine and estuarine sulfate-limited sediments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banat I. M., Lindström E. B., Nedwell D. B., Balba M. T. Evidence for coexistence of two distinct functional groups of sulfate-reducing bacteria in salt marsh sediment. Appl Environ Microbiol. 1981 Dec;42(6):985–992. doi: 10.1128/aem.42.6.985-992.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebl H., Pfennig Growth of sulfate-reducing bacteria with sulfur as electron acceptor. Arch Microbiol. 1977 Feb 4;112(1):115–117. doi: 10.1007/BF00446664. [DOI] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W. Estimations of bacterial growth rates in natural waters. J Bacteriol. 1969 Jul;99(1):156–160. doi: 10.1128/jb.99.1.156-160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Wiebe W. J. Tracer analysis of methanogenesis in salt marsh soils. Appl Environ Microbiol. 1980 Apr;39(4):877–881. doi: 10.1128/aem.39.4.877-881.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanbroek H. J., Pfennig N. Oxidation of short-chain fatty acids by sulfate-reducing bacteria in freshwater and in marine sediments. Arch Microbiol. 1981 Jan;128(3):330–335. doi: 10.1007/BF00422540. [DOI] [PubMed] [Google Scholar]
- Laanbroek H. J., Veldkamp H. Microbial interactions in sediment communities. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 11;297(1088):533–550. doi: 10.1098/rstb.1982.0059. [DOI] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A., Mays E. L., Tiedje J. M. Carbon and electron flow in mud and sandflat intertidal sediments at delaware inlet, nelson, new zealand. Appl Environ Microbiol. 1980 Apr;39(4):686–694. doi: 10.1128/aem.39.4.686-694.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Role of sulfate reduction versus methanogenesis in terminal carbon flow in polluted intertidal sediment of waimea inlet, nelson, new zealand. Appl Environ Microbiol. 1981 Aug;42(2):252–258. doi: 10.1128/aem.42.2.252-258.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S. Methane production in shallow-water, tropical marine sediments. Appl Microbiol. 1975 Oct;30(4):602–608. doi: 10.1128/am.30.4.602-608.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol. 1982 Dec;44(6):1270–1276. doi: 10.1128/aem.44.6.1270-1276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Electron donors utilized by sulfate-reducing bacteria in eutrophic lake sediments. Appl Environ Microbiol. 1981 Jul;42(1):116–121. doi: 10.1128/aem.42.1.116-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J., Christensen D., Jørgensen B. B. Volatile Fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981 Jul;42(1):5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUEPER H. G., SCHLEGEL H. G. SULPHUR METABOLISM IN THIORHODACEAE. I. QUANTITATIVE MEASUREMENTS ON GROWING CELLS OF CHROMATIUM OKENII. Antonie Van Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Ward D. M. Substrates for sulfate reduction and methane production in intertidal sediments. Appl Environ Microbiol. 1983 Jan;45(1):193–199. doi: 10.1128/aem.45.1.193-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


