Abstract
The combined effects of cholesterol, a major cell membrane component, and the lipid second messenger diacylglycerol on the activity of protein kinase C (PK-C) and the structure of phosphatidylcholine/phosphatidylserine bilayers were investigated using specific PK-C assays and 2H NMR. Whereas the classical activation of PK-C was observed as an effect of diacylglycerol, in the absence of this second messenger, cholesterol did not affect PK-C activity. A novel effect of amplified PK-C activation was observed in the presence of both cholesterol and diacylglycerol concentrations within the physiological range of each of these components. 2H NMR results suggest that this phenomenon is due to cholesterol- and diacylglycerol-induced increased propensity of the lipids to adopt nonbilayer phases, effectively destabilizing the bilayer structure. The magnitude of the effect was a function of cholesterol concentration, implying that laterally separated cell membrane domains with distinct cholesterol concentrations have the capacity to differ in their sensitivity to extracellular stimuli.
INTRODUCTION
It has become generally accepted that plasma cell membranes are not homogeneous structures, but contain laterally separated regions with distinct lipid and protein compositions (1–7). Although the precise nature of such regions is hotly debated (see reviews (2,8,9)), the signature characteristics of a subset of such regions (“rafts”) are increased cholesterol and sphingomyelin content with corresponding changes in physical properties, such as lipid chain order, membrane thickness, and diffusion coefficients of lipid and protein molecules contained in the rafts (1,2,9). Gómez-Moutón et al. (10) further demonstrated that there are multiple types of rafts in cells, each with a specific complement of proteins, and that the rafts played an important role in the migration of T lymphocytes. Membrane rafts have additionally been implicated in a diverse set of cellular processes, including signal transduction (1), protein sequestration (11), heat shock response (12), and intracellular trafficking (13).
A number of membrane-associated proteins are known to be affected by the physical state and associated physicochemical parameters of the membrane, including protein kinase C (PK-C) (14–17), phospholipase-A2 (18), G proteins (19), colipase (20), and phospholipase C (PL-C) (21). The ubiquitous membrane-associated signal transduction enzyme, PK-C, is a relatively well-studied example. PK-C is involved in many cellular processes, including differentiation, apoptosis, migration, adhesion, angiogenesis (22), and tumorigenesis (23). Conventional isoforms of PK-C require diacylglycerol, phosphatidylserine (PS), and Ca2+ for their activation. Furthermore, a number of studies from ours and others' laboratories have demonstrated that the activity of PK-C is also modulated by the physicochemical parameters of lipid bilayers and perturbations of these parameters induced by diacylglycerols and other second messengers, such as ceramides and unesterified fatty acids, thereby providing an additional signal-integrating mechanism (14,15,24–27).
Diacylglycerols are produced primarily by the hydrolysis of phosphatidylinositol 4,5-bisphosphate by PL-C to produce diacylglycerol and inositol 1,4,5-trisphosphate (28,29). Additional means of producing diacylglycerols are the degradation of phosphatidylcholine by the phospholipase D/PAP pathway (30) or the PL-C pathway (28). Beyond their role as a second messenger in activating PK-C, diacylglycerols also activate other proteins which contain a C1 domain, including RasGRP, Chimaerin, and Munc family members (31). There are at least 50 distinct molecular species of diacylglycerol in mammalian cells (32,33), from polyunsaturated to saturated variants, the polyunsaturated and mono-unsaturated variants being the most biologically relevant. In this study, we used diolein, a mono-unsaturated variant.
Cholesterol is an important constituent of cell membranes, and has wide-ranging effects on the physico-chemical properties of membranes, including the average acyl chain order (34), gel-to-liquid crystalline transition temperatures (35), lateral diffusion rates (36), permeability of membranes to ions, and the average area of lipid molecules (37). The modulation of membrane properties by cholesterol is also related to the diverse biological effects of cholesterol, some of which have been observed upon the removal of cholesterol by Mβ-cyclodextrin or other means, including the function of G protein-coupled receptors (38), cytotoxicity of AβP in Alzheimer's disease (39), and many others (40).
Here we extend our previous studies of the modulation by membrane structure of membrane-associated signal transduction proteins by examining the effects of cholesterol, diolein (a diacylglycerol), and sphingomyelin on PK-C activity and structure of phosphatidylcholine (PC)/PS bilayers using PK-C assays and 2H NMR. The results show that cholesterol is capable of modulating the activity of membrane-associated signal transduction proteins, in addition to its previously known membrane-ordering effects.
MATERIALS AND METHODS
Materials
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-L-serine] (POPS), 1-perdeuteriopalmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (d31-POPC), 1-perdeuteriopalmitoyl-2-oleoyl-sn-glycero-3-[Phospho-L-Serine] (d31-POPS), bovine brain sphingomyelin, cholesterol, and diolein were purchased from Avanti Polar Lipids (Alabaster, AL). Human recombinant PK-C-α was purchased from Invitrogen (Carlsbad, CA), Whatman P81 filter paper from Whatman (Kent, UK), adenine triphosphate (ATP), Histone III-S, and morpholinopropanesulfonic acid (MOPS) from Sigma (St. Louis, MO). The γ-32P ATP was obtained from PerkinElmer (Boston, MA).
Methods
PK-C assays
A quantity of 300 nmol of POPC/POPS (4:1 mol/mol) in chloroform were aliquoted into test tubes in triplicate along with diolein, cholesterol, and sphingomyelin as the sample required. The chloroform was then evaporated under dry N2 and then completely evaporated in a <1 mTorr vacuum overnight. The lipids were then hydrated with 50 μL of buffer (2 mM MgCl2, 25 μM CaCl2, 20 mM MOPS, pH 7.4), and underwent at least five freeze/thaw cycles to ensure proper mixing of lipids. The final lipid concentration of 3.75 mM is well within the lipid cofactor saturation regime (41). Thus, if the multilamellarity of the lipid vesicles is changed by the addition of diacylglycerol resulting in a change of the effective lipid concentration, PK-C activity will not be affected. The lipids were then heated to 30°C in a water bath and 20 μL of substrate was added (2 mM MgCl2, 25 μM CaCl2, 20 mM MOPS, 0.83 μg/mL Histone III-S, 20 μM ATP, 7 μCi γ-32P ATP, pH 7.4 (final concentrations)). The low concentrations of CaCl2, histone, and ATP were chosen to have minimal effects on bilayer structure (25,42). To start the reaction, 10 ng PK-C in 10 μL buffer was added to the mixture. After 5 min of incubation, the reaction was stopped by depositing 55 μL of the mixture on P81 filter paper circles which were then washed four times in 500 mL of 50 mM NaCl, each wash lasting 30 min. The filter paper circles were then rinsed with acetone, and placed in scintillation vials with 2 mL scintillation fluid and counted on a scintillation counter (Beckman Coulter, Fullerton, CA).
2H NMR measurements
The lipids (in chloroform) for the sample, including diolein, cholesterol, and sphingomyelin as appropriate were aliquoted into cuvettes. The lipid mixture included 5 mg d31-POPC or d31-POPS, which replaced an equivalent molar amount of unlabeled lipid in the mixture. The chloroform was then evaporated under a stream of dry N2 and 20:1 benzene/methanol (v:v) was added to resuspend the lipids, a commonly used method which has previously been shown to yield uniform distribution (43). The resultant mixture was then lyophilized, hydrated in deuterium-depleted buffer (65 mM MgCl2, 40 mM MOPS pH 7.4) at 1:2 w/w (lipid/buffer), sealed, and underwent five freeze/thaw cycles. The MgCl2 concentration was calculated to maintain the same ratio of PS bound to Mg2+ to unbound PS in both PK-C assays and NMR experiments, as explained in our previous publication (14).
Spectra were acquired on a Varian Inova 500 spectrometer at 11.74 T (corresponding to a 76.77 MHz 2H frequency; Varian, Cary, NC) with a high power 2H probe after equilibrating at the run temperature for 20 min using the standard quadrupole echo sequence (90x–τ–90y) with a refocusing time (τ) of 60 μs, a 90° pulse of 3.5 μs, a 200 ms recycle time, and a 500 kHz spectral width. Transients were acquired until the signal/noise ratio reached an acceptable level, which typically took on the order of 10,000 transients. Acquired transients were then interpolated using bicubic splines and left-shifted to the top of the echo (44) (software available at http://rzlab.ucr.edu/nmr), and analyzed using Felix 1.0 (http://apache.org). Numeric deconvolution (or dePakeing) was performed using the fast Fourier transform method (45,46).
RESULTS
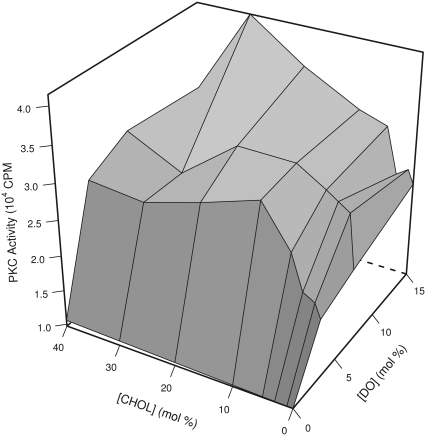
The dependence of PK-C activity on the presence of cholesterol and diolein is depicted in Fig. 1, which shows the results of a single typical experiment, with concentrations of diolein between 0 and 15 mol % and concentrations of cholesterol between 0 and 40 mol %. Each individual vertex of the graph is an average of three replicates, the standard errors of which are on the order of those given in Fig. 2. We observed the classical effect of diolein-activating PK-C at all concentrations of cholesterol tested. This effect is easily discernible on the right-front cube face at 0 mol % cholesterol. In the absence of diolein, cholesterol does not affect PK-C activity, as is apparent from the left-front cube face. A novel effect of cholesterol amplifying the increase of PK-C activity by diolein is seen in the first altitude gain on the left side that corresponds to an increase from 0 to 10 mol % cholesterol. The amplification by cholesterol is apparent at low physiological concentrations of diolein (3 mol %) and within the range of physiological cholesterol concentrations (20–40 mol %), and persists at higher concentrations of diolein. There is a peak of PK-C activity at 30 mol % cholesterol and 15% diolein, corresponding to a 1.7-fold increase relative to the level without cholesterol; further addition of cholesterol leads to decreased PK-C activity.
FIGURE 1.
Amplification by cholesterol of the activation of PK-C by diacylglycerol. PK-C activity of samples containing various concentrations of cholesterol (CHOL) and diolein (DO), with diolein concentration increasing from the center front to the right rear, and cholesterol concentration increasing from the center front to the left rear. Every intersection is the mean of PK-C activity from triplicate samples from a single experiment, with error bars corresponding to standard error omitted for clarity. See Fig. 2 for representative error sizes. The shading indicates the average activity of each quadrilateral: lighter shading indicates higher activity.
FIGURE 2.
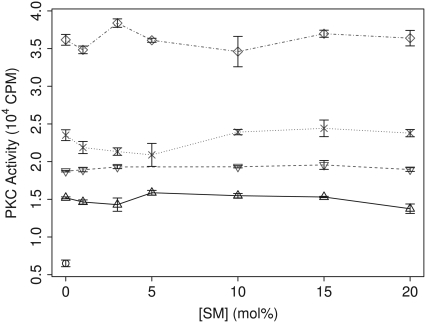
Dependence of PK-C activity on sphingomyelin. PK-C activity of samples containing sphingomyelin (SM) amounts ranging from 0 mol % to 20 mol % without PK-C (○), with PK-C (▵), with 3 mol % diolein (∇), with 10 mol % diolein (×), and with 10 mol % diolein and 25 mol % cholesterol (⋄).
Since sphingomyelin is another major component of cholesterol and sphingomyelin-enriched membrane domains, we next examined the effect of sphingomyelin on PK-C activity within the same range of concentrations of cholesterol and diolein given above. However, as seen in Fig. 2, there was no significant effect of sphingomyelin on PK-C activity.
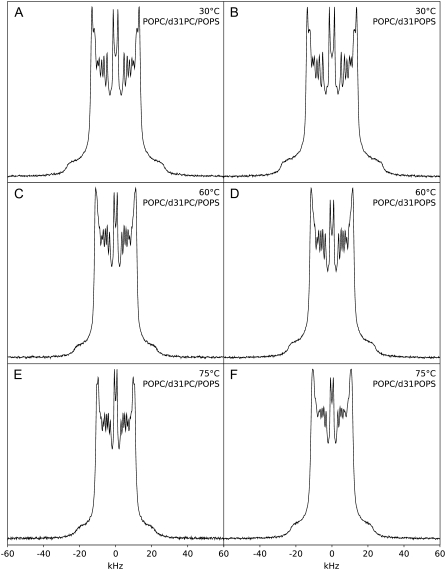
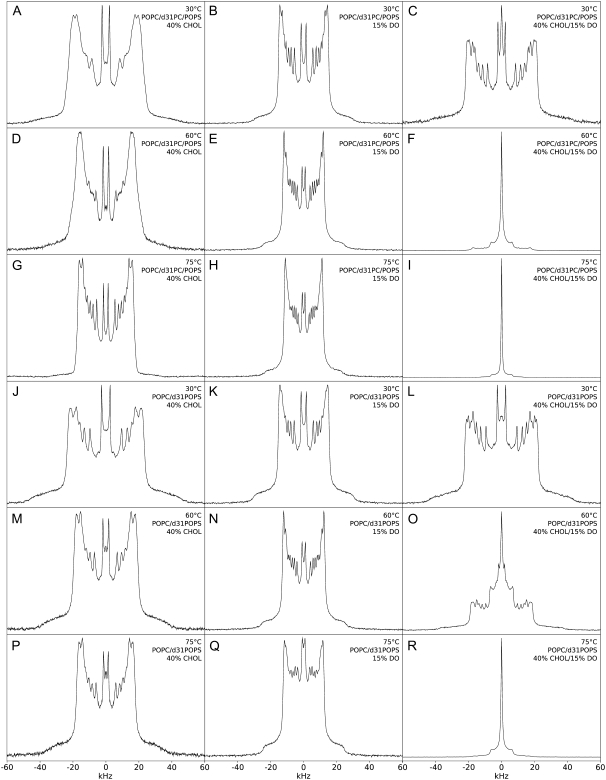
The dependence of PK-C activity on the physico-chemical parameters of the lipid cofactors has been previously demonstrated by us and others (14,15,26,47–52). To understand the mechanism behind the amplification by cholesterol of the PK-C activation by diolein we next used 2H NMR to examine samples with the same composition as those in PK-C assays, except a fraction of POPC or POPS was substituted with the corresponding acyl chain per-deuterated nonperturbing labels: d31-POPC or d31-POPS, respectively. Fig. 3 shows the 2H NMR spectra of a 4:1 mol/mol mixture of POPC and POPS at various temperatures. We have used a 4:1 ratio of POPC to POPS as this is representative of the ratio of PC to PS in animal membranes (53–55). The spectra are well resolved, and indicative of the liquid crystalline bilayer phase with no other phases present at the temperatures shown. These spectra are consistent with those previously observed by us (56) and others (35,57). The spectra correspond to a powder pattern generated by the acyl-chain deuterons at different positions in the acyl chains and all orientations relative to the external magnetic field. We have observed a maximum of nine peaks, whose position corresponds to the perpendicular orientation of the specific deuteron magnetic moments relative to the external magnetic field. The assignment of these peaks to the specific positions of the acyl chains was done according to Morrison and Bloom (57), and reflects the relationship of the quadrupole splittings and the order parameters of the corresponding C-D bonds (SCD, Eq. 1, (58)),
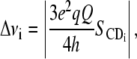 |
(1) |
where 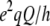 or νQ is the static quadrupole coupling constant, 167 kHz (59), and Δνi is the peak-to-peak quadrupole splitting of the corresponding deuteron. The order parameters at the top of the acyl chains, close to the phospholipid headgroups, have the highest values—reflected in the largest quadrupole splittings (the plateau region) with the top-seven position with the identical order parameters corresponding to the wide-end shoulders of the spectra (Figs. 3–5) and the plateau on the SCD plots (Fig. 6). The order parameters monotonously decrease along the chains with the terminal methyl corresponding to the least-ordered, well-resolved inner pair of symmetrical peaks (Figs. 3–5.) The SCD profiles of d31-POPC and d31-POPS are slightly different (Fig. 6) for mixtures which do not contain diolein. The individual peaks of d31-POPC spectra (Fig. 4, A, D, and G; and Fig. 5, A and D) are broader than those of d31-POPS spectra (Fig. 4, J, M, and P; and Fig. 5, J and M). This observation is consistent with previously reported differences between cholesterol interactions with PC or PS (60,61), although the exact mechanism for this difference is not clear (61). We also cannot completely exclude the possibility of lateral phase separation of domains slightly enriched in cholesterol in the absence of diolein. This peak broadening is abolished by addition of diacylglycerol, making the d31-POPC and d31-POPS spectra virtually superimposable (see corresponding panels on Figs. 4 and 5), and thus is unlikely to have an effect on PK-C activity which requires the presence of diacylglycerol. The SCD profiles of d31-POPC and d31-POPS differ slightly in their behavior upon the addition of diolein to mixtures containing cholesterol: diolein slightly increases the ordering of d31-POPC containing membranes, while it has the opposite effect on d31-POPS, though the effects are small.
or νQ is the static quadrupole coupling constant, 167 kHz (59), and Δνi is the peak-to-peak quadrupole splitting of the corresponding deuteron. The order parameters at the top of the acyl chains, close to the phospholipid headgroups, have the highest values—reflected in the largest quadrupole splittings (the plateau region) with the top-seven position with the identical order parameters corresponding to the wide-end shoulders of the spectra (Figs. 3–5) and the plateau on the SCD plots (Fig. 6). The order parameters monotonously decrease along the chains with the terminal methyl corresponding to the least-ordered, well-resolved inner pair of symmetrical peaks (Figs. 3–5.) The SCD profiles of d31-POPC and d31-POPS are slightly different (Fig. 6) for mixtures which do not contain diolein. The individual peaks of d31-POPC spectra (Fig. 4, A, D, and G; and Fig. 5, A and D) are broader than those of d31-POPS spectra (Fig. 4, J, M, and P; and Fig. 5, J and M). This observation is consistent with previously reported differences between cholesterol interactions with PC or PS (60,61), although the exact mechanism for this difference is not clear (61). We also cannot completely exclude the possibility of lateral phase separation of domains slightly enriched in cholesterol in the absence of diolein. This peak broadening is abolished by addition of diacylglycerol, making the d31-POPC and d31-POPS spectra virtually superimposable (see corresponding panels on Figs. 4 and 5), and thus is unlikely to have an effect on PK-C activity which requires the presence of diacylglycerol. The SCD profiles of d31-POPC and d31-POPS differ slightly in their behavior upon the addition of diolein to mixtures containing cholesterol: diolein slightly increases the ordering of d31-POPC containing membranes, while it has the opposite effect on d31-POPS, though the effects are small.
FIGURE 3.
2H NMR spectra of mixtures of POPC/POPS 4:1 w/w with d31-POPC (A, C, and E) or d31-POPS (B, D, and F) at 30 (A and B), 60 (C and D), and 75°C (E and F).
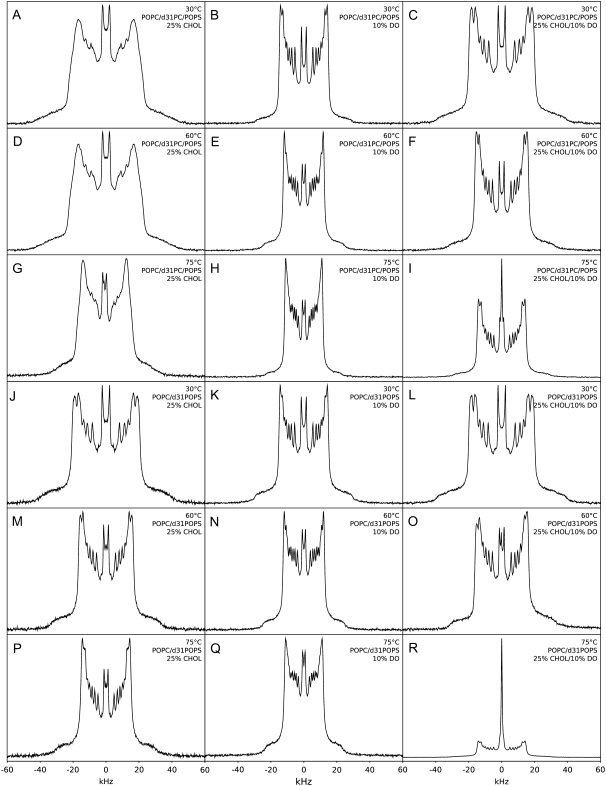
FIGURE 4.
2H NMR spectra of mixtures of POPC/POPS 4:1 w/w with d31-POPC (A–I) or d31-POPS (J–R) and 25% cholesterol and/or 10% diolein at 30, 60, and 75°C.
FIGURE 5.
2H NMR spectra of mixtures of POPC/POPS 4:1 w/w with d31-POPC (A–I) or d31-POPS (J–R) and 40% cholesterol and/or 15% diolein at 30, 60, and 75°C.
FIGURE 6.
SCD plot of d31-POPC (A) and d31-POPS (B) labeled spectra at 30°C. Missing points correspond to locations where peaks could not be unambiguously assigned. (Labels: ○, 4:1 POPC/POPS alone; ▵, with 40% cholesterol; +, with 15% diolein; ×, with 40% cholesterol and 15% diolein; ⋄ with 25% cholesterol; ▿ with 10% diolein; ⊠, with 25% cholesterol and 10% diolein.)
Whereas in the absence of cholesterol addition of diolein increased the order parameters of both PC and PS, consistent with our previous results (14,15), in the presence of cholesterol this effect became insignificant (Fig. 6). These results are similar to our previous results obtained with addition of d62-DPPC or d62-DPPS to bilayers composed of bovine liver PC extracts, DPPC, and DPPS (25).
The average area-per-lipid-molecule of d31-POPC and d31-POPS calculated using the SCD values and the mean torque model (and methyl volume formula given therein) (62) results in areas at 30°C of 60.6 Å2 for mixtures without cholesterol or diolein, decreasing to 50.3 Å2 at 40 mol % cholesterol and 15 mol % diolein. These areas are smaller than those reported by Greenwood et al. (63), but are similar to the areas calculated by simulation (64) and experiment (65).
Fig. 4 shows the 2H NMR spectra of POPC and POPS (4:1 mol/mol) labeled with d31-POPC (Fig. 4, A–I) or d31-POPS (J-R) mixed with 25 mol % cholesterol, 10 mol % diolein, or both at 30, 60, and 75°C (as indicated). With 25% cholesterol, the spectra are substantially less well resolved and significantly wider (more ordered) than the corresponding spectra without cholesterol, indicative of the presence of the liquid-ordered phase (lo) (56,66,67) at the temperatures shown (Fig. 4, A, D, and G). DePakeing (45,46) of this and other cholesterol-containing spectra did not result in a significant improvement of the resolution and specifically did not allow us to discern any additional peaks (not shown). With 10% diolein, the spectra are slightly wider than the corresponding spectra without diolein, better resolved, and are in a bilayer phase at all temperatures shown (Fig. 4, B, E, and H). This bilayer-ordering effect of diolein and other diacylglycerols has been shown by us previously (15,47). When 10% diolein and 25% cholesterol are added together, however, an isotropic, nonbilayer phase (central peak) is observed at 75°C (Fig. 4, I and R). This nonbilayer phase is not observed at lower temperatures (Fig. 4, C and F). These spectra are also significantly better resolved than the corresponding spectra without diolein, perhaps corresponding to a decrease in the range of available local cholesterol concentrations. An alternative possibility is the partial orientation of diolein-containing spectra, most likely due to the high-field strength of the instrument used. However, this does not alter the splittings at the 90° position, and consequently does not alter the SCD (68). The samples containing d31-POPS behave similarly, with slightly higher order parameters consistent with previously published results (56). Although Fig. 4 R (25% cholesterol, 10% diolein, d31-POPS) shows a higher intensity central peak than the corresponding d31-POPC labeled spectra (Fig. 4 I) the relative areas (corresponding to the distribution of the labeled lipids between the phases) are similar, indicating that there is no significant difference in d31-POPC and d31-POPS distribution among the lipid phases.
The amplification by cholesterol of the inducement of nonbilayer lipid phases by diolein is even more pronounced upon increase of cholesterol concentration to 40 mol %, which is within the range of physiological cholesterol concentrations in the plasma membrane (53–55) (Fig. 5). Increasing cholesterol to 40 mol % also results in further widening of the spectra, as compared to 25 mol % cholesterol (Fig. 4), consistent with the ordering effect of cholesterol.
With 15% diolein (DO) (Fig. 5, B, E, and H), the spectra are still well resolved (in the absence of cholesterol) and slightly more ordered than the corresponding spectra with 10% diolein (Fig. 4 B, E, and H). Spectra with both 40% cholesterol and 15% diolein show the presence of an isotropic phase (small vesicles or micelles), coexisting with a liquid-ordered phase, which becomes more pronounced at higher temperatures (Fig. 5, F, I, O, and R).
DISCUSSION
In our previous studies we have demonstrated that diacylglycerols, alone or in combination with other lipid second-messenger molecules, such as unesterified fatty acids or ceramides, activate PK-C and perturb (generally by destabilizing) lipid bilayer structure (14,15,25,26). Furthermore, we reached the conclusion that the combined bilayer perturbation induced by diacylglycerols and other second messengers is the driving force in their combined effects on PK-C activity. In this study, we have continued our previous work by studying such important cell membrane components as cholesterol and sphingomyelin. To examine the effect of lipid bilayer structure on PK-C activity, we have used 2H NMR, which is able to determine the chain order and phase composition of membranes nearly identical to those used for PK-C assays while minimizing perturbation due to the label, as often occurs when using fluorescent or electron spin resonance probes (69). This allows us to infer relationships between the physicochemical parameters of the membrane, such as the propensity to form nonbilayer phases, and the level of PK-C activation measured by the PK- C assay.
Cholesterol modulates activities of membrane-associated enzymes
We have found profound combined effects of diacylglycerol and cholesterol on both PK-C activity and lipid bilayer structure. The presence of cholesterol in combination with diacylglycerol amplifies both the diacylglycerol-induced PK-C activity (Fig. 1) and the propensity of the lipids to adopt nonbilayer phases, which is pronounced even at 25% cholesterol with 10% diolein (Fig. 4, C, F, and I), and closely corresponds to the maximum PK-C activity (Fig. 1). This supports the previously presented hypothesis (24,26,49) that this propensity is a factor promoting PK-C activity. We (and others) have consistently detected increases in PK-C activity induced by a variety of lipophilic compounds (14,15,25,50–52), which by themselves do not activate PK-C, but destabilize bilayers by increasing their propensity to adopt nonbilayer lipid phases. Conversely, compounds that decrease this propensity (i.e., seem to stabilize bilayers) attenuate PK-C activity (50–52).
The simplest explanation of these observations is that the bilayer instability observed as the increased propensity to adapt nonbilayer lipid phases is an important factor in PK-C activation. Also, consistent with our previous findings (14,15), the actual presence of nonbilayer phases at the temperature of PK-C assays, exhibited by 40% cholesterol and 15% diolein (Fig. 5, C, F, and I), is detrimental to PK-C activity, as can be seen on Fig. 1. It has previously been shown that the addition of cholesterol to model membranes can promote phase separation in phosphatidylethanolamine (PE)/PS (70), the cubic nonbilayer phase in dioleoyl-phosphatidylcholine-containing mixtures (71) (although these phases were only seen transiently with POPC/cholesterol mixtures), and the formation of isotropic vesicles in bovine brain-PC/d31-POPC/cholesterol/diolein mixtures at 60°C (56).
Our results at 40 mol % cholesterol, which show a significant increase in order parameters induced by cholesterol, are consistent with the bilayers being in the liquid-ordered (lo) phase. Different conclusions were reached recently in a similar system: Filippor et al. (72) concluded from the low activation energy and linear decrease of lipid diffusion coefficient with the addition of cholesterol that the bilayers are in liquid-disordered (ld) phase at this temperature. However, work by Aussenac et al. (73) using 2H NMR of cholesterol-2H5 indicates that mixtures of POPC containing >22% cholesterol are in lo; these conclusions are also in agreement with published phase diagrams of Veatch and Keller (74), de Almeida et al. (75), and our previous work (56).
Our results are consistent with a few reports that showed increased activity of PK-C in the presence of cholesterol (76–78). Cholesterol sulfate has also previously been implicated in the activation of certain PK-C isozymes (79). Furthermore, cholesterol has also been shown to be an important modulator of other membrane-associated proteins. A study by Escribá and co-workers with G-proteins has shown that the addition of cholesterol favors the binding of G-proteins to membranes at lower concentrations (10–20 mol %) but then decreases at higher concentrations (30–50 mol %) (19), qualitatively similar to the biphasic response seen in the PK-C assays presented here, where increasing cholesterol beyond a certain point at high diolein decreases the activity of PK-C. Cholesterol has also been shown to reduce the lag phase of the activation of PL-C, presumably by increasing the frustration in the membrane, and thereby allowing less hindered access of PL-C to lipids in the membrane (21).
Diacylglycerols affect activities of membrane-associated enzymes
Diacylglycerols, such as diolein, are important membrane components and are required cofactors for the activation of classical and novel PK-C isozymes (80). Diacylglycerols increase the affinity of PK-C for PS-containing membranes by two orders of magnitude (17), both by the binding of PK-C's C1 domain to diacylglycerol and by increasing the intrinsic negative curvature of the bilayer (81), in so doing increasing the propensity of the bilayer to form nonbilayer phases. In addition to their effect on PK-C, diacylglycerols also modulate the activities of other enzymes which have the diacylglycerol-binding C1 domain, including the Ras guanyl-releasing protein family of nucleotide exchange factors, protein kinase D, and serine threonine kinases (82) as well as some membrane-associated enzymes which do not have C1 domains, such as colipase (20).
Sphingomyelin does not affect PK-C activity or bilayer structure
Sphingomyelin, a component of lipid rafts which is found predominantly in the outer leaflet (83,84), does not have a significant modulating effect on PK-C activity, the order parameters of PC or PS in the lipid bilayer, or the presence of nonbilayer lipid phases under the conditions of this study. This was rather unexpected, as it has been previously reported that sphingomyelin stabilizes PE/PS mixtures against the HII transition (70).
Physiological relevance of membrane composition
The concentrations of cholesterol, diacylglycerols, sphingomyelin, and phospholipids vary among cell types (53,55,85), and more importantly, vary within the plasma membranes themselves. Membrane domains (of which “lipid rafts” are a subset) are regions of distinct lipid and protein composition (54,86,87) with correspondingly distinct physicochemical parameters. We have examined a range of cholesterol, sphingomyelin, and diacylglycerol concentrations to understand the physical behavior of model membranes corresponding to modulated PK-C activity. We used a maximum of 20 mol % sphingomyelin, which is lower than the concentration of sphingomyelin found in the canonical lipid-raft mixture (33 mol %) (88). However, it encompasses the range of the concentrations found within the inner leaf of the plasma membrane (53,89). In the case of diacylglycerols, concentrations on the order of a few mol % can activate PK-C. However, the diacylglycerols which activate PK-C are produced in close proximity to the signaling stimulus by PL-C with localized, transiently high concentration. This concentration is likely to be larger than the overall concentration of diacylglycerol (which can reach 10 mol % in some transformed cells (27)), making the range of diolein concentrations utilized here appropriate. As for cholesterol, it has been noted that many domains of the plasma membrane have concentrations of cholesterol which differ from the bulk membrane (53,90). Traditionally, most of the membrane surface was considered to be in a continuous ld phase, with smaller, unconnected domains of higher cholesterol levels in lo phase (giving rise to the term “rafts”). However, given that the overall concentration of cholesterol present in most cells is on the order of 30–50 mol % and that the lo phase is often present at concentrations at 20–25 mol % cholesterol, it is more likely that the lo forms the continuous phase, with the smaller domains with reduced cholesterol being in ld phase (91) (we suggest calling these ld domains “ponds”).
CONCLUSIONS
The ability of cholesterol to amplify the activation of PK-C and the destabilization of the bilayer phase by diacylglycerol makes it clear that membrane regions with varying concentrations of cholesterol can have a significant modulating effect upon the function of membrane-associated signal transduction proteins that are sensitive to bilayer strain. In the case of PK-C and its activator diacylglycerol, regions of greater cholesterol content will be more sensitive to the production of diacylglycerol than regions with lesser cholesterol content.
It has been reported that cholesterol concentrations in the raft and nonraft regions are 34 mol % and 15 mol %, respectively (53). This change in cholesterol content corresponds to ≈1.5 increase in PK-C activity (Fig. 1). Thus, raft-associated receptors will be 50% more efficient in activating PK-C than nonassociated receptors, suggesting that different classes of membrane domains will have distinct signal transduction characteristics due to their variable cholesterol content, in addition to other properties, such as the selective association of membrane proteins with specific membrane domains. These results, in conjunction with previous studies, can modify the conceptual understanding of cell membranes from performing primarily structural functions to active participants in transmembrane signal transduction.
Acknowledgments
We thank Dr. D. B. Borchardt for his help in optimizing 2H NMR experiments.
Editor: Paul H. Axelsen.
References
- 1.Pike, L. J. 2003. Lipid rafts: bringing order to chaos. J. Lipid Res. 44:655–667. [DOI] [PubMed] [Google Scholar]
- 2.Zeyda, M., and T. M. Stulnig. 2006. Lipid Rafts & Co.: an integrated model of membrane organization in T cell activation. Prog. Lipid Res. 45:187–202. [DOI] [PubMed] [Google Scholar]
- 3.Vereb, G., J. Szölläsi, J. Matkó, P. Nagy, T. Farkas, L. Vigh, L. Mátyus, T. A. Waldmann, and S. Damjanovich. 2003. Dynamic, yet structured: the cell membrane three decades after the Singer-Nicolson model. Proc. Natl. Acad. Sci. USA. 100:8053–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sepúlveda, M. R., M. Berrocal-Carrillo, M. Gasset, and A. M. Mata. 2006. The plasma membrane Ca2+-ATPase isoform 4 is localized in lipid rafts of cerebellum synaptic plasma membranes. J. Biol. Chem. 281:447–453. [DOI] [PubMed] [Google Scholar]
- 5.Floto, R. A., M. R. Clatworthy, K. R. Heilbronn, D. R. Rosner, P. A. MacAry, A. Rankin, P. J. Lehner, W. H. Ouwehand, J. M. Allen, N. A. Watkins, and K. G. C. Smith. 2005. Loss of function of a lupus-associated FcγRIIb polymorphism through exclusion from lipid rafts. Nat. Med. 11:1056–1058. [DOI] [PubMed] [Google Scholar]
- 6.Kim, B.-W., H.-J. Choo, J.-W. Lee, J.-H. Kim, and Y.-G. Ko. 2004. Extracellular ATP is generated by ATP synthase complex in adipocyte lipid rafts. Exp. Mol. Med. 36:476–485. [DOI] [PubMed] [Google Scholar]
- 7.Wisniewska, A., J. Draus, and W. K. Subczynski. 2003. Is a fluid-mosaic model of biological membranes fully relevant? Studies on lipid organization in model and biological membranes. Cell. Mol. Biol. Lett. 8:147–159. [PubMed] [Google Scholar]
- 8.Edidin, M. 2003. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 32:257–283. [DOI] [PubMed] [Google Scholar]
- 9.London, E. 2005. How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells. Biochim. Biophys. Acta. 1746:203–220. [DOI] [PubMed] [Google Scholar]
- 10.Gómez-Moutón, C., E. M. Jose Luis Abad, R. A. Lacalle, E. Gallardo, I. I. Sonia Jiménez-Baranda, A. Bernad, S. M. Nes, and C. Martínez. 2001. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc. Natl. Acad. Sci. USA. 98:9642–9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvaraj, V., A. Asano, D. E. Buttke, J. L. McElwee, J. L. Nelson, C. A. Wolff, T. Merdiushev, M. W. Fornés, A. W. Cohen, M. P. Lisanti, G. H. Rothblat, G. S. Kopf, and A. J. Travis. 2006. Segregation of micron-scale membrane sub-domains in live murine sperm. J. Cell. Physiol. 206:636–646. [DOI] [PubMed] [Google Scholar]
- 12.Vigh, L., P. V. Escribá, A. Sonnleitner, M. Sonnleitner, S. Piotto, B. Maresca, I. Horváth, and J. L. Harwood. 2005. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog. Lipid Res. 44:303–344. [DOI] [PubMed] [Google Scholar]
- 13.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39. [DOI] [PubMed] [Google Scholar]
- 14.Huang, H. W., E. M. Goldberg, and R. Zidovetzki. 1999. Ceramides modulate protein kinase C activity and perturb the structure of phosphatidylcholine/phosphatidylserine bilayers. Biophys. J. 77:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg, E. M., and R. Zidovetzki. 1998. Synergistic effects of diacylglycerols and fatty acids on membrane structure and protein kinase C activity. Biochemistry. 37:5623–5632. [DOI] [PubMed] [Google Scholar]
- 16.Jiménez-Monreal, A. M., F. J. Aranda, V. Micol, P. S.-P. Nera, A. de Godos, and J. C. Gómez-Fernández. 1999. Influence of the physical state of the membrane on the enzymatic activity and energy of activation of protein kinase Cα. Biochemistry. 38:7747–7754. [DOI] [PubMed] [Google Scholar]
- 17.Medkova, M., and W. Cho. 1998. Differential membrane-binding and activation mechanisms of protein kinase C-α and -ɛ. Biochemistry. 37:4892–4900. [DOI] [PubMed] [Google Scholar]
- 18.Huang, H. W., E. M. Goldberg, and R. Zidovetzki. 1996. Ceramide induces structural defects into phosphatidylcholine bilayers and activates phospholipase A2. Biochem. Biophys. Res. Commun. 220:834–838. [DOI] [PubMed] [Google Scholar]
- 19.Escribá, P. V., A. Ozaita, C. Ribas, A. Miralles, E. Fodor, T. Farkas, and J. A. García-Sevilla. 1997. Role of lipid polymorphism in G protein-membrane interactions: nonlamellar-prone phospholipids and peripheral protein binding to membranes. Proc. Natl. Acad. Sci. USA. 94:11375–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugar, I. P., N. K. Mizuno, M. M. Momsen, and H. L. Brockman. 2001. Lipid lateral organization in fluid interfaces controls the rate of colipase association. Biophys. J. 81:3387–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz-Argüello, M. B., F. M. Goñi, and A. Alonso. 1998. Phospholipase C hydrolysis of phospholipids in bilayers of mixed lipid compositions. Biochemistry. 37:11621–11628. [DOI] [PubMed] [Google Scholar]
- 22.Nakashima, S. 2002. Protein kinase C alpha (PKC α): regulation and biological function. J. Biochem. (Tokyo). 132:669–675. [DOI] [PubMed] [Google Scholar]
- 23.Griner, E. M., and M. G. Kazanietz. 2007. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer. 7:281–294. [DOI] [PubMed] [Google Scholar]
- 24.Senisterra, G., and R. M. Epand. 1993. Role of membrane defects in the regulation of the activity of protein kinase C. Arch. Biochem. Biophys. 300:378–383. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg, E. M., D. S. Lester, D. B. Borchardt, and R. Zidovetzki. 1994. Effects of diacylglycerols and Ca2+ on structure of phosphatidylcholine/phosphatidylserine bilayers. Biophys. J. 66:382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg, E. M., and R. Zidovetzki. 1997. Effects of dipalmitoylglycerol and fatty acids on membrane structure and protein kinase C activity. Biophys. J. 73:2603–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dibble, A. R., A. K. Hinderliter, J. J. Sando, and R. L. Biltonen. 1996. Lipid lateral heterogeneity in phosphatidylcholine/phosphatidylserine/diacylglycerol vesicles and its influence on protein kinase C activation. Biophys. J. 71:1877–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakelam, M. J. 1998. Diacylglycerol—when is it an intracellular messenger? Biochim. Biophys. Acta. 1436:117–126. [DOI] [PubMed] [Google Scholar]
- 29.Rhee, S. G. 2001. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70:281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Exton, J. H. 1997. New developments in phospholipase D. J. Biol. Chem. 272:15579–15582. [DOI] [PubMed] [Google Scholar]
- 31.Springett, G. M., H. Kawasaki, and D. R. Spriggs. 2004. Non-kinase second-messenger signaling: new pathways with new promise. Bioessays. 26:730–738. [DOI] [PubMed] [Google Scholar]
- 32.Pessin, M. S., J. J. Baldassare, and D. M. Raben. 1990. Molecular species analysis of mitogen-stimulated 1,2-diglycerides in fibroblasts. Comparison of α-thrombin, epidermal growth factor, and platelet-derived growth factor. J. Biol. Chem. 265:7959–7966. [PubMed] [Google Scholar]
- 33.Pettitt, T. R., and M. J. Wakelam. 1993. Bombesin stimulates distinct time-dependent changes in the sn-1,2-diradylglycerol molecular species profile from Swiss 3T3 fibroblasts as analyzed by 3,5-dinitrobenzoyl derivitization and HPLC separation. Biochem. J. 289:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ipsen, J. H., O. G. Mouritsen, and M. Bloom. 1990. Relationships between lipid membrane area, hydrophobic thickness, and acyl-chain orientational order. The effects of cholesterol. Biophys. J. 57:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]
- 36.Lindblom, G., G. Orädd, and A. Filippov. 2006. Lipid lateral diffusion in bilayers with phosphatidylcholine, sphingomyelin and cholesterol. An NMR study of dynamics and lateral phase separation. Chem. Phys. Lipids. 141:179–184. [DOI] [PubMed] [Google Scholar]
- 37.Leathes, J. 1925. Rôle of fats in vital phenomena. Lancet. 208:853–856. [Google Scholar]
- 38.Pucadyil, T. J., and A. Chattopadhyay. 2006. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog. Lipid Res. 45:295–333. [DOI] [PubMed] [Google Scholar]
- 39.Arispe, N., and M. Doh. 2002. Plasma membrane cholesterol controls the cytotoxicity of Alzheimer's disease AβP (1–40) and (1–42) peptides. FASEB J. 16:1526–1536. [DOI] [PubMed] [Google Scholar]
- 40.Zidovetzki, R., and I. Levitan. 2007. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta. 1768:1311–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sando, J. J., and O. I. Chertihin. 1996. Activation of protein kinase C by lysophosphatidic acid: dependence on composition of phospholipid vesicles. Biochem. J. 317:583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberg, E. M., D. B. Borchardt, and R. Zidovetzki. 1998. Effects of histone and diolein on the structure of phosphatidylcholine/phosphatidylserine or phosphatidylcholine/phosphatidylglycerol bilayers. Eur. J. Biochem. 258:722–728. [DOI] [PubMed] [Google Scholar]
- 43.Bach, D., E. Wachtel, N. Borochov, G. Senisterra, and R. M. Epand. 1992. Phase behavior of heteroacid phosphatidylserines and cholesterol. Chem. Phys. Lipids. 63:105–113. [Google Scholar]
- 44.Ronemus, A. D., R. L. Vold, and R. R. Vold. 1986. Deuterium quadrupole echo NMR spectroscopy. II. Artifact suppression. J. Magn. Reson. 70:416–426. [Google Scholar]
- 45.Sternin, E., M. Bloom, and A. MacKay. 1983. De-Pakeing of NMR spectra. J. Magn. Reson. 55:274–282. [Google Scholar]
- 46.McCabe, M. A., and S. R. Wassal. 1995. Fast-Fourier-transform dePaking. J. Mag. Res. Ser. B. 106:80–82. [Google Scholar]
- 47.De Boeck, H., and R. Zidovetzki. 1989. Effects of diacylglycerols on the structure of phosphatidylcholine bilayers: a 2H and 31P NMR study. Biochemistry. 28:7439–7446. [DOI] [PubMed] [Google Scholar]
- 48.Escribá, P. V., M. Sastre, and J. A. García-Sevilla. 1995. Disruption of cellular signaling pathways by daunomycin through destabilization of nonlamellar membrane structures. Proc. Natl. Acad. Sci. USA. 92:7595–7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Epand, R. M. 1997. Studies of membrane physical properties and their role in biological function. Biochem. Soc. Trans. 25:1073–1079. [DOI] [PubMed] [Google Scholar]
- 50.Epand, R. M. 1987. The relationship between the effects of drugs on bilayer stability and on protein kinase C activity. Chem. Biol. Interact. 63:239–247. [DOI] [PubMed] [Google Scholar]
- 51.Epand, R. M., A. R. Stafford, J. J. Cheetham, R. Bottega, and E. H. Ball. 1988. The relationship between the bilayer to hexagonal phase transition temperature in membranes and protein kinase C activity. Biosci. Rep. 8:49–54. [DOI] [PubMed] [Google Scholar]
- 52.Epand, R. M., and D. S. Lester. 1990. The role of membrane biophysical properties in the regulation of protein kinase C activity. Trends Pharmacol. Sci. 11:317–320. [DOI] [PubMed] [Google Scholar]
- 53.Pike, L. J., X. Han, K.-N. Chung, and R. W. Gross. 2002. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 41:2075–2088. [DOI] [PubMed] [Google Scholar]
- 54.Baron, C. B., and R. F. Coburn. 2004. Smooth muscle raft-like membranes. J. Lipid Res. 45:41–53. [DOI] [PubMed] [Google Scholar]
- 55.Tewes, B., and H. Galla. 2001. Lipid polarity in brain capillary endothelial cells. Endothelium. 8:207–220. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong, D. L., D. B. Borchardt, and R. Zidovetzki. 2002. Synergistic perturbation of phosphatidylcholine/sphingomyelin bilayers by diacylglycerol and cholesterol. Biochem. Biophys. Res. Commun. 296:806–812. [DOI] [PubMed] [Google Scholar]
- 57.Morrison, C., and M. Bloom. 1994. Orientation dependence of 2H nuclear magnetic resonance spin-lattice relaxation in phospholipid and phospholipid:cholesterol systems. J. Chem. Phys. 101:749–763. [Google Scholar]
- 58.Davis, J., and K. Jeffrey. 1977. The temperature dependence of chain disorder in potassium palmitate-water. A deuterium NMR study. Chem. Phys. Lipids. 20:87–104. [Google Scholar]
- 59.Burnett, L. J., and B. H. Muller. 1971. Deuterium quadrupole coupling constants in three solid deuterated paraffin hydrocarbons: C2D6, C4D10, C6D14. J. Chem. Phys. 55:5829–5831. [Google Scholar]
- 60.Leventis, R., and J. R. Silvius. 2001. Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophys. J. 81:2257–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Epand, R. M., A. D. Bain, B. G. Sayer, D. Bach, and E. Wachtel. 2002. Properties of mixtures of cholesterol with phosphatidylcholine or with phosphatidylserine studied by 13C magic angle spinning nuclear magnetic resonance. Biophys. J. 83:2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petrache, H. I., S. W. Dodd, and M. F. Brown. 2000. Area per lipid and acyl length distributions in fluid phosphatidylcholines determined by 2H NMR spectroscopy. Biophys. J. 79:3172–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenwood, A. I., S. Tristram-Nagle, and J. F. Nagle. 2006. Partial molecular volumes of lipids and cholesterol. Chem. Phys. Lipids. 143:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Róg, T., and M. Pasenkiewicz-Gierula. 2006. Cholesterol effects on a mixed-chain phosphatidylcholine bilayer: a molecular dynamics simulation study. Biochimie. 88:449–460. [DOI] [PubMed] [Google Scholar]
- 65.Smaby, J. M., M. M. Momsen, H. L. Brockman, and R. E. Brown. 1997. Phosphatidylcholine acyl unsaturation modulates the decrease in interfacial elasticity induced by cholesterol. Biophys. J. 73:1492–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bloom, M., E. Evans, and O. G. Mouritsen. 1991. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q. Rev. Biophys. 24:293–397. [DOI] [PubMed] [Google Scholar]
- 67.Clarke, J. A., A. J. Heron, J. M. Seddon, and R. V. Law. 2006. The diversity of the liquid ordered (Lo) phase of phosphatidylcholine/cholesterol membranes: a variable temperature multinuclear solid-state NMR and x-ray diffraction study. Biophys. J. 90:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pott, T., and E. J. Dufourc. 1995. Action of melittin on the DPPC-cholesterol liquid-ordered phase: a solid state 2H-and 31P-NMR study. Biophys. J. 68:965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vogel, A., H. A. Scheidt, and D. Huster. 2003. The distribution of lipid attached spin probes in bilayers: application to membrane protein topology. Biophys. J. 85:1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf, C., K. Koumanov, B. Tenchov, and P. J. Quinn. 2001. Cholesterol favors phase separation of sphingomyelin. Biophys. Chem. 89:163–172. [DOI] [PubMed] [Google Scholar]
- 71.Tenchov, B. G., R. C. MacDonald, and D. P. Siegel. 2006. Cubic phases in phosphatidylcholine-cholesterol mixtures: cholesterol as membrane “fusogen”. Biophys. J. 91:2508–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Filippov, A., G. Orädd, and G. Lindblom. 2003. The effect of cholesterol on the lateral diffusion of phospholipids in oriented bilayers. Biophys. J. 84:3079–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aussenac, F., M. Tavares, and E. J. Dufourc. 2003. Cholesterol dynamics in membranes of raft composition: a molecular point of view from 2H and 31P solid-state NMR. Biochemistry. 42:1383–1390. [DOI] [PubMed] [Google Scholar]
- 74.Veatch, S. L., and S. L. Keller. 2005. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 1746:172–185. [DOI] [PubMed] [Google Scholar]
- 75.de Almeida, R. F. M., A. Fedorov, and M. Prieto. 2003. Sphingomyelin/ phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 85:2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niggli, V., A. V. Meszaros, C. Oppliger, and S. Tornay. 2004. Impact of cholesterol depletion on shape changes, actin reorganization, and signal transduction in neutrophil-like HL-60 cells. Exp. Cell Res. 296:358–368. [DOI] [PubMed] [Google Scholar]
- 77.Bolen, E. J., and J. J. Sando. 1992. Effect of phospholipid unsaturation on protein kinase C activation. Biochemistry. 31:5945–5951. [DOI] [PubMed] [Google Scholar]
- 78.Slater, S. J., M. B. Kelly, F. J. Taddeo, C. Ho, E. Rubin, and C. D. Stubbs. 1994. The modulation of protein kinase C activity by membrane lipid bilayer structure. J. Biol. Chem. 269:4866–4871. [PubMed] [Google Scholar]
- 79.Kuroki, T., T. Ikuta, M. Kashiwagi, S. Kawabe, M. Ohba, N. Huh, K. Mizuno, S. Ohno, E. Yamada, and K. Chida. 2000. Cholesterol sulfate, an activator of protein kinase C mediating squamous cell differentiation: a review. Mutat. Res. 462:189–195. [DOI] [PubMed] [Google Scholar]
- 80.Newton, A. C., and J. E. Johnson. 1998. Protein kinase C: a paradigm for regulation of protein function by two membrane-targeting modules. Biochim. Biophys. Acta. 1376:155–172. [DOI] [PubMed] [Google Scholar]
- 81.Das, S., and R. P. Rand. 1986. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 25:2882–2889. [DOI] [PubMed] [Google Scholar]
- 82.Kazanietz, M. G. 2002. Novel “nonkinase” phorbol ester receptors: the C1 domain connection. Mol. Pharmacol. 61:759–767. [DOI] [PubMed] [Google Scholar]
- 83.Calderón, R. O., and G. H. DeVries. 1997. Lipid composition and phospholipid asymmetry of membranes from a Schwann cell line. J. Neurosci. Res. 49:372–380. [PubMed] [Google Scholar]
- 84.Verkleij, A., R. Zwaal, B. Roelofsen, P. Comfurius, D. Kastelijn, and L. van Deenen. 1973. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim. Biophys. Acta. 323:178–193. [DOI] [PubMed] [Google Scholar]
- 85.Boesze-Battaglia, K., and R. Schimmel. 1997. Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. J. Exp. Biol. 200:2927–2936. [DOI] [PubMed] [Google Scholar]
- 86.Pike, L. J. 2004. Lipid rafts: heterogeneity on the high seas. Biochem. J. 378:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Babiychuk, E. B., and A. Draeger. 2006. Biochemical characterization of detergent-resistant membranes: a systematic approach. Biochem. J. 397:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Veatch, S. L., and S. L. Keller. 2003. A closer look at the canonical “raft mixture” in model membrane studies. Biophys. J. 84:725–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaus, K., M. Rodriguez, K. R. Ruberu, I. Gelissen, T. M. Sloane, L. Kritharides, and W. Jessup. 2005. Domain-specific lipid distribution in macrophage plasma membranes. J. Lipid Res. 46:1526–1538. [DOI] [PubMed] [Google Scholar]
- 90.Drobnik, W., H. Borsukova, A. Böttcher, A. Pfeiffer, G. Liebisch, G. J. Schötz, H. Schindler, and G. Schmitz. 2002. Apo AI/ABCA1-dependent and HDL3-mediated lipid efflux from compositionally distinct cholesterol-based microdomains. Traffic. 3:268–278. [DOI] [PubMed] [Google Scholar]
- 91.Holowka, D., J. A. Gosse, A. T. Hammond, X. Han, P. Sengupta, N. L. Smith, A. Wagenknecht-Wiesner, M. Wu, R. M. Young, and B. Baird. 2005. Lipid segregation and IgE receptor signaling: a decade of progress. Biochim. Biophys. Acta. 1746:252–259. [DOI] [PubMed] [Google Scholar]