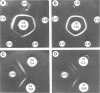
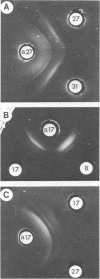
Abstract
Because of the scarcity of literature on the successful use of serological methods for differentiation of Rhizobium meliloti isolates, the objectives of this study were to provide a rationale for selecting isolates to which antisera could be raised and to appraise the suitability of published methods of preparing R. meliloti antigens for the serological identification of field isolates. We used one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis to develop protein profiles of eight field isolates and one commercial inoculant strain of R. meliloti in order to choose candidates that were either identical or distinctly different from each other for the production of antisera. The serological methods of tube agglutination and gel immunodiffusion complemented the sodium dodecyl sulfate-polyacrylamide gel electrophoresis method of identification. On the basis of their agglutination titers and gel immunodiffusion analysis, the isolates were placed in five serogroups which were identical to the groupings based on protein profiles. Antigenic characteristics of gel immunodiffusion antigens were influenced by the composition of the growth medium, sonication of whole-cell antigens, and the addition of Formalin. We recommend that careful attention be given to the effects of varying antigen preparation procedures when analyzing R. meliloti so that experimental protocols do not complicate the results. The wide range of homologous-antiserum titers observed for the nine isolates indicates different inherent degrees of immunogenicity of R. meliloti which cannot be predicted before serum production. The sodium dodecyl sulfate-polyacrylamide gel electrophoresis method is a useful tool for screening a collection of R. meliloti isolates to better ensure that strain-specific antisera representative of different types of organisms will be obtained.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DATE R. A., DECKER A. M. MINIMAL ANTIGENIC CONSTITUTION OF 28 STRAINS OF RHIZOBIUM JAPONICUM. Can J Microbiol. 1965 Feb;11:1–8. doi: 10.1139/m65-001. [DOI] [PubMed] [Google Scholar]
- DUDMAN W. F. IMMUNE DIFFUSION ANALYSIS OF THE EXTRACELLULAR SOLUBLE ANTIGENS OF TWO STRAINS OF RHIZOBIUM MELILOTI. J Bacteriol. 1964 Sep;88:782–794. doi: 10.1128/jb.88.3.782-794.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbins L. N. The preparation of antigens of Rhizobium meliloti by ultrasonic disruption: an anomaly. Can J Microbiol. 1967 Oct;13(10):1375–1378. doi: 10.1139/m67-184. [DOI] [PubMed] [Google Scholar]
- Humphrey B. A., Vincent J. M. The effect of calcium nutrition on the production of diffusible antigens by Rhizobium trifolii. J Gen Microbiol. 1965 Oct;41(1):109–118. doi: 10.1099/00221287-41-1-109. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leps W. T., Roberts G. P., Brill W. J. Use of Two-Dimensional Polyacrylamide Electrophoresis to Demonstrate that Putative Rhizobium Cross-Inoculation Mutants Actually Are Contaminants. Appl Environ Microbiol. 1980 Feb;39(2):460–462. doi: 10.1128/aem.39.2.460-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Brill W. J. Diversity and Dynamics of Indigenous Rhizobium japonicum Populations. Appl Environ Microbiol. 1980 Nov;40(5):931–938. doi: 10.1128/aem.40.5.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes V. G., Schmidt E. L. Population Densities of Rhizobium japonicum Strain 123 Estimated Directly in Soil and Rhizospheres. Appl Environ Microbiol. 1979 May;37(5):854–858. doi: 10.1128/aem.37.5.854-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., Leps W. T., Silver L. E., Brill W. J. Use of two-dimensional polyacrylamide gel electrophoresis to identify and classify Rhizobium strains. Appl Environ Microbiol. 1980 Feb;39(2):414–422. doi: 10.1128/aem.39.2.414-422.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinghamer E. A. Effectiveness of Rhizobium as modified by mutation for resistance to antibiotics. Antonie Van Leeuwenhoek. 1967;33(2):121–136. doi: 10.1007/BF02045542. [DOI] [PubMed] [Google Scholar]
- Sinha R. C., Peterson E. A. Homologous serological analysis of Rhizobium meliloti strains by immunodiffusion. Can J Microbiol. 1980 Sep;26(9):1157–1161. doi: 10.1139/m80-191. [DOI] [PubMed] [Google Scholar]
- Verbruggen Quantitative immunoelectrophoretic methods: a literature survey. Clin Chem. 1975 Jan;21(1):5–43. [PubMed] [Google Scholar]
- Vincent J. M., Humphrey B. A. Modification of the antigenic surface of Rhizobium trifolii by a deficiency of calcium. J Gen Microbiol. 1968 Dec;54(3):397–405. doi: 10.1099/00221287-54-3-397. [DOI] [PubMed] [Google Scholar]
- Wilson M. H., Humphrey B. A., Vincent J. M. Loss of agglutinating specificity in stock cultures of Rhizobium meliloti. Arch Microbiol. 1975 Apr 7;103(2):151–154. doi: 10.1007/BF00436342. [DOI] [PubMed] [Google Scholar]