Short abstract
A comprehensive survey of alternate splicing across 42 eukaryotes so as to gain insight into how spliceosomal introns are recognized.
Abstract
Background
Variations in transcript splicing can reveal how eukaryotes recognize intronic splice sites. Retained introns (RIs) commonly appear when the intron definition (ID) mechanism of splice site recognition inconsistently identifies intron-exon boundaries, and cassette exons (CEs) are often caused by variable recognition of splice junctions by the exon definition (ED) mechanism. We have performed a comprehensive survey of alternative splicing across 42 eukaryotes to gain insight into how spliceosomal introns are recognized.
Results
All eukaryotes we studied exhibit RIs, which appear more frequently than previously thought. CEs are also present in all kingdoms and most of the organisms in our analysis. We observe that the ratio of CEs to RIs varies substantially among kingdoms, while the ratio of competing 3' acceptor and competing 5' donor sites remains nearly constant. In addition, we find the ratio of CEs to RIs in each organism correlates with the length of its introns. In all 14 fungi we examined, as well as in most of the 9 protists, RIs far outnumber CEs. This differs from the trend seen in 13 multicellular animals, where CEs occur much more frequently than RIs. The six plants we analyzed exhibit intermediate proportions of CEs and RIs.
Conclusion
Our results suggest that most extant eukaryotes are capable of recognizing splice sites via both ID and ED, although ED is most common in multicellular animals and ID predominates in fungi and most protists.
Background
Intron splicing occurs in all domains of life, but the splicing methods employed and the frequencies of splicing vary among organisms. Bacteria and archaea lack the spliceosomal pathway and splice infrequently via self-splicing introns. Among unicellular eukaryotes, there is substantial range in splicing frequency [1,2]. Many early-branching eukaryotes, including the protists Giardia, Cryptosporidia, Trypanosoma, Entamoeba, and Trichomonas, have few or no introns. Only 5% of genes are spliced in Saccharomyces cerevisiae [3], a yeast, while the average number of introns per gene among other fungi is generally low (with a few noteworthy exceptions). Their average intron density ranges from just over one in Schizosaccharomyces pombe to approximately five in Cryptococcus neoformans [4]. Protists have similarly low rates of splicing. In contrast, multicellular animals often have large numbers of introns (over seven per gene in vertebrates), while plants have intermediate numbers of introns (approximately four per gene in Oryza sativa and Arabidopsis thaliana).
The number of introns and recognized splice sites may vary between individual mRNA transcripts of a single gene, giving rise to the phenomena of splice variation and alternative splicing. In this paper we use 'splice variation' to describe any difference in intron processing, reserving the term 'alternative splicing' for splice variation that is regulated and functionally significant. Observed splice variation is a combination of programmed alternative splicing events and splicing errors. Functional alternative splicing may result from various causes, including ontogenic changes and environmental stimuli. As the number of genes in an organism is not well correlated with its complexity, alternative splicing may provide an additional layer of regulation that permits greater complexity in higher organisms [5]. Multicellular organisms may generate different splice forms of the same gene in different tissues, or even within different cells in the same tissue [5,6]. More recently, it has also been demonstrated that alternative splicing can vary between individuals in a heritable manner [7].
Splice variants can be divided into four broad categories: retained introns (RIs), cassette exons (CEs), competing 5' splice sites, and competing 3' splice sites. CEs are the predominant form of splice variation in multicellular eukaryotes [8-10], whereas RIs are more frequent in multicellular plants such as A. thaliana and O. sativa [11-13], as well as the fungus Cryptococcus and in yeast [14-17].
The profile of splice variants in a given organism is likely influenced by the mechanisms it uses to identify and process splice sites. In eukaryotes, it has been proposed that the spliceosome recognizes splice sites in pairs, either across the intron (intron definition (ID)) or across the exon (exon definition (ED)) [9]. In ID, splice sites on either side of an intron are recognized as a unit, while in ED, splice sites on either side of an exon are recognized as a unit. Experiments in both yeast and Drosophila have shown that when splice sites are presumably recognized by ID, mutating a single splice site disrupts splicing of the intron adjacent to the mutation. This leads to an RI, but has no effect on the splicing of nearby introns [17,18] (Figure 1). In contrast, when splice sites are presumably recognized by ED, mutating a single splice site affects not only the splicing of the intron adjacent to the mutation, but also the intron on the other side of the exon adjacent to the mutation. This causes cassette exons to be skipped [19,20]. Therefore, it is believed that with ID, splicing errors are more likely to result in RIs, while with ED, splicing errors are more likely to result in CEs. ID and ED are not mutually exclusive; in Drosophila melanogaster, ID and ED have been shown to operate within a single mRNA [21].
Figure 1.
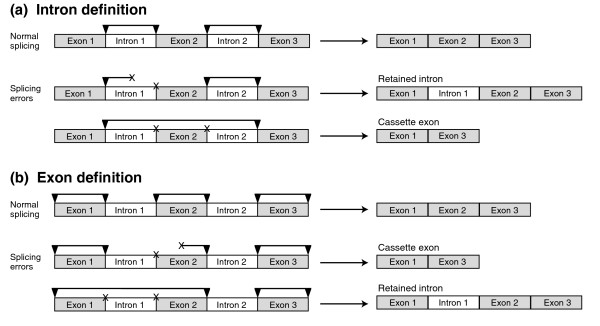
Effects of splicing errors under the intron definition (ID) and exon definition (ED) models. Arrowheads connected by horizontal bars illustrate the paired recognition of splice sites. (a) When splice sites are recognized in pairs across introns by ID, an error at a single splice site (marked 'x') prevents the removal of an intron, leading to a RI. Under ID, two adjacent splice sites must be mis-spliced, and the splicing machinery must operate over a greater distance, to generate a CE. (b) In the ED model, splice sites are recognized in pairs across exons. An error at a single splice site results in a CE. Obtaining an RI via ED requires coordinated mis-recognition of two adjacent splice sites over a longer distance. Observed RIs can be parsimoniously explained by ID-mediated splicing, while observed CEs likely indicate splicing via ED.
The method used to recognize splice sites has been associated with restrictions on exon and intron length. Recognition of splice sites with ED appears to constrain exon length [20,22], while recognition with ID limits intron length [18,23]. Fox-Walsh et al. [24] suggest that splice site recognition across the intron in D. melanogaster ceases at lengths greater than around 200-250 bp. A review of previous studies suggests that phylogenetic trends in exon and intron length may be correlated with the relative occurrence of RIs and CEs and the use of ID or ED for splice junction recognition [16,18,20,23,24]. However, previous results have been limited in their phylogenetic scope.
In this paper, we report a comprehensive survey of splice variants in 42 eukaryotic organisms. Our survey covers a wide phylogenetic range, including 13 multicellular animals, 6 plants, 14 fungi, and 9 protists. We observe variation across major phylogenetic groups in the representation of RIs and CEs among splice variants that is consistent with variation in the mode of splice site recognition (ID or ED) used by these groups. We infer that groups with a high ratio of RIs to CEs (fungi and protists) operate predominantly by ID, while groups with a low ratio of RIs to CEs (multicellular animals) operate predominantly by ED. In organisms with evidence of both RIs and CEs (thus, employing both ID and ED), CEs are shorter than constitutive exons (exons that show no evidence of splice variation), and RIs are shorter than constitutive introns, suggesting that splice mechanisms are closely tied to gene structure.
Results
To assess splice variation in eukaryotes, we selected 42 organisms with genome assemblies and large numbers of publicly available expressed sequence tags (ESTs; Table 1), spanning the plants, fungi, protists and multicellular animals. We aligned ESTs to genome assemblies and constructed transcript fragments, examined all loci where the EST data indicated two or more overlapping non-compatible transcripts, and labeled every instance of splice variation. Table 2 shows the numbers of ESTs for each organism, as well as the numbers of transcripts and loci constructed. Table 3 lists the splice variants we found. A complete list of the locations of predicted sites of splice variation, as well as control introns and exons that show no splice variation despite high EST coverage, is available on the Broad Institute's ftp site [25].
Table 1.
Genomes and ESTs used in the analysis
| Genome name | Kingdom | Reference | ESTs |
|---|---|---|---|
| Danio rerio | Animal | Assembly Zv6, Sanger Institute [66] | Genbank 06/15/07 |
| Takifugu rubripes | Animal | [67] | Genbank 06/22/07 |
| Branchistoma floridae | Animal | JGI [60] (v1.0) | Genbank 06/22/07 |
| Ciona savignyi | Animal | Broad Institute [58] (ci1.0) | Genbank 06/15/07 |
| Ciona intestinalis | Animal | [68] | Genbank 06/15/07 |
| Nematostella vectensis | Animal | [69] | Genbank 06/22/07 |
| Strongylocentrotus purpuratus | Animal | [70] | Genbank 06/27/07 |
| Drosophila melanogaster | Animal | [71] | Genbank 06/15/07 |
| Aedes aegypti | Animal | [72] | Vectorbase [61,73] |
| Anopheles gambiae. | Animal | [74] | Vectorbase [61,73] |
| Apis mellifera | Animal | [75] | Genbank 06/18/07 |
| Caenorhabditis elegans | Animal | [76] | Genbank 02/1/07 |
| Schistosoma mansoni | Animal | TIGR/JCVI [59] | Genbank 06/22/07 |
| Rhizopus oryzae | Fungi | Broad Institute [58] | Genbank 06/15/07 + Broad Institute [58] |
| Cryptococcus neoformans JEC21 | Fungi | [15] | Genbank 06/19/07 |
| Ustilago maydis | Fungi | [77] | Genbank 06/15/07 |
| Schizosaccharomyces pombe | Fungi | [78] | Genbank 01/14/08 |
| Saccharomyces cerevisiae | Fungi | [79] | Genbank 01/14/08 |
| Neurospora crassa | Fungi | [80] | Genbank 06/15/07 |
| Magnaporthe grisea 70-15 | Fungi | [81] | Genbank 06/15/07 |
| Stagnospora nodorum | Fungi | Broad Institute [58] | Genbank 06/15/07 |
| Aspergillus flavus NRRL3357 | Fungi | TIGR/JCVI [59], GenBank AAIH00000000 | Genbank 06/15/07 |
| Aspergillus nidulans | Fungi | [82] | Genbank 06/15/07 |
| Histoplasma capsulatum | Fungi | Broad Institute [58] | Genbank 06/15/07 |
| Coccidioides posadasii | Fungi | TIGR/JCVI [59] | Genbank 06/15/07 |
| Coccidioides immitis RS | Fungi | Broad Institute [58] | Genbank 06/15/07 |
| Sclerotinia sclerotiorum 1980 | Fungi | Broad Institute [58] | Genbank 06/15/07 + Broad Institute [58] |
| Dictyostelium discoideum | Protist | [83] | Genbank 06/15/07 |
| Populus trichocarpa | Plant | [84] | Genbank 06/18/07 |
| Arabidopsis thaliana | Plant | [85] | Genbank 06/16/07 |
| Oryza sativa | Plant | [86] | Genbank 06/15/07 |
| Physcomitrella patens | Plant | JGI [60] (v1.1) | Genbank 06/28/07 |
| Chlamydomonas reinhardtii | Plant | JGI [60] (v3.0) | Genbank 06/18/07 |
| Ostreococcus lucimarinus | Plant | [87] | JGI [60] |
| Phytophthora infestans | Protist | Broad Institute [58] (C Nusbaum, personal communication) | Genbank 06/15/07 |
| Phytophthora sojae | Protist | [88] | Genbank 06/15/07 |
| Plasmodium yoelii | Protist | [89] | Genbank 06/15/07 |
| Plasmodium falciparum 3D7 | Protist | [90] | Genbank 06/19/07 |
| Paramecium tetraurelia | Protist | [91] | Genbank 06/15/07 |
| Tetrahymena thermophila | Protist | [92] | Genbank 06/19/07 |
| Entamoeba hystolitica | Protist | [4] | Genbank 06/18/07 |
| Phaeodactylum tricornutum | Protist | JGI [60] | Genbank 06/28/07 |
Table 2.
Numbers of ESTs, transcripts, and loci
| Kingdom | Total ESTs* | Spliced. Filtered ESTs† | Transcripts‡ | Loci§ | |
|---|---|---|---|---|---|
| D. rerio | Animal | 1350,105 | 556,175 | 76,066 | 33,338 |
| T. rubripes | Animal | 26,069 | 14,197 | 5,890 | 4,988 |
| B. floridae | Animal | 277,538 | 57,883 | 15,784 | 12,054 |
| C. savignyi | Animal | 84,302 | 24,196 | 7,027 | 8,382 |
| C. intestinalis | Animal | 686,396 | 334,137 | 31,794 | 15,108 |
| N. vectensis | Animal | 16,619 | 6,206 | 4,609 | 4,294 |
| S. purpuratus | Animal | 141,833 | 24,770 | 23,256 | 20,773 |
| D. melanogaster | Animal | 532,557 | 242,235 | 25,241 | 14,965 |
| A. aegypti | Animal | 303,409 | 120,523 | 15,878 | 11,389 |
| A. gambiae | Animal | 216,617 | 95,607 | 13,511 | 9,835 |
| A. mellifera | Animal | 78085 | 32,860 | 9,581 | 7,800 |
| C. elegans | Animal | 346,064 | 219,812 | 28,438 | 21,304 |
| S. mansoni | Animal | 158,841 | 55,392 | 14,494 | 10,137 |
| R. oryzae | Fungi | 25,393 | 12,238 | 3,263 | 3,052 |
| C. neoformans | Fungi | 59,041 | 46,693 | 8,173 | 6,361 |
| U. maydis | Fungi | 39,308 | 11,236 | 1,289 | 1,109 |
| S. pombe | Fungi | 5,574 | 843 | 274 | 269 |
| S. cerevisiae | Fungi | 32,653 | 2,251 | 259 | 245 |
| N. crassa | Fungi | 28,089 | 7,577 | 1,571 | 1,495 |
| M. grisea | Fungi | 53,102 | 14,563 | 3,229 | 2,795 |
| S. nodorum | Fungi | 15,973 | 5,925 | 1,637 | 1,537 |
| A. flavus | Fungi | 20,371 | 8,495 | 3,004 | 2,772 |
| A. nidulans | Fungi | 16,848 | 5,499 | 2,240 | 2,090 |
| H. capsulatum | Fungi | 26,389 | 2,334 | 850 | 950 |
| C. posadasii | Fungi | 54,217 | 30,604 | 6,769 | 5,296 |
| C. immitis | Fungi | 65,754 | 32,162 | 6,484 | 5,133 |
| S. sclerotiorum | Fungi | 65,884 | 30,203 | 4,314 | 3,704 |
| D. discoideum | Protist | 155,032 | 46,116 | 4,687 | 4,246 |
| P. trichocarpa | Plant | 89,943 | 38,299 | 11,462 | 9,377 |
| A. thaliana | Plant | 1,276,692 | 350,380 | 35,856 | 23,412 |
| O. sativa | Plant | 977,774 | 374,397 | 43,265 | 25,610 |
| P. patens | Plant | 194,822 | 106,309 | 21,962 | 15,402 |
| C. reinhardtii | Plant | 167,641 | 72,903 | 11,353 | 8,514 |
| O. lucimarinus | Plant | 19,200 | 1,043 | 328 | 304 |
| P. infestans | Protist | 94,091 | 17,381 | 4,762 | 4,104 |
| P. sojae | Protist | 28,357 | 7,418 | 2,125 | 2,012 |
| P. yoelii | Protist | 13,925 | 2,863 | 1,019 | 931 |
| P. falciparum | Protist | 21,349 | 3,928 | 1,417 | 1,219 |
| P. tetraurelia | Protist | 86,070 | 44,772 | 11,423 | 10,258 |
| T. thermophila | Protist | 56,543 | 21,073 | 6,035 | 5,540 |
| E. histolytica | Protist | 20,404 | 599 | 174 | 166 |
| P. tricornutum | Protist | 89,139 | 14,576 | 3,325 | 2,937 |
*Number of raw ESTs before filtering. †Number of ESTs aligned after applying our set of filters, containing at least one splice site (see Materials and methods). ‡Number of 'transcripts' constructed from the ESTs. §Number of 'loci' (overlapping clusters of transcripts) (genes with 1+ splice site).
Table 3.
Types of splice variants observed
| Retained introns | ||||||||
|---|---|---|---|---|---|---|---|---|
| Kingdom | No. of splice variants | Cassette exons | Discarding unspliced ESTs | Keeping unspliced ESTs* | Competing 5' sites | Competing 3' sites | (CE fraction) | |
| D. rerio | Animal | 6,137 | 2,475 | 1,176 | 2,324 | 931 | 1,555 | 0.68 |
| T. rubripes | Animal | 201 | 52 | 47 | 79 | 50 | 52 | 0.53 |
| B. floridae | Animal | 812 | 516 | 26 | 123 | 121 | 149 | 0.95 |
| C. savignyi | Animal | 181 | 93 | 18 | 64 | 22 | 48 | 0.84 |
| C. intestinalis | Animal | 2,779 | 834 | 907 | 2,084 | 394 | 644 | 0.48 |
| N. vectensis | Animal | 108 | 54 | 8 | 33 | 21 | 25 | 0.87 |
| S. purpuratus | Animal | 284 | 147 | 13 | 50 | 63 | 61 | 0.92 |
| D. melanogaster | Animal | 2,163 | 453 | 712 | 2,343 | 442 | 556 | 0.39 |
| A. aegypti | Animal | 983 | 280 | 284 | 606 | 186 | 233 | 0.50 |
| A. gambiae | Animal | 882 | 207 | 283 | 635 | 190 | 202 | 0.42 |
| A. mellifera | Animal | 561 | 153 | 124 | 338 | 135 | 149 | 0.55 |
| C. elegans | Animal | 1,589 | 361 | 452 | 828 | 285 | 491 | 0.44 |
| S. mansoni | Animal | 1,236 | 235 | 599 | 974 | 143 | 259 | 0.28 |
| R. oryzae | Fungi | 47 | 0 | 30 | 55 | 5 | 12 | 0 |
| C. neoformans | Fungi | 1,091 | 18 | 768 | 1,117 | 101 | 204 | 0.02 |
| U. maydis | Fungi | 85 | 8 | 31 | 105 | 16 | 30 | 0.21 |
| S. pombe | Fungi | 3 | 0 | 3 | 13 | 0 | 0 | 0 |
| S. cerevisiae | Fungi | 9 | 0 | 2 | 42 | 3 | 4 | 0 |
| N. crassa | Fungi | 20 | 2 | 8 | 30 | 5 | 5 | 0.20 |
| M. grisea | Fungi | 151 | 5 | 86 | 194 | 24 | 36 | 0.05 |
| S. nodorum | Fungi | 36 | 2 | 21 | 37 | 5 | 8 | 0.09 |
| A. flavus | Fungi | 162 | 3 | 124 | 302 | 13 | 22 | 0.02 |
| A. nidulans | Fungi | 100 | 1 | 74 | 216 | 8 | 17 | 0.01 |
| H. capsulatum | Fungi | 50 | 1 | 33 | 65 | 6 | 10 | 0.03 |
| C. posadasii | Fungi | 950 | 15 | 648 | 1,259 | 120 | 167 | 0.02 |
| C. immitis | Fungi | 861 | 8 | 542 | 1,035 | 131 | 180 | 0.01 |
| S. sclerotiorum | Fungi | 323 | 9 | 210 | 419 | 49 | 55 | 0.04 |
| D. discoideum | Protist | 107 | 6 | 33 | 98 | 29 | 39 | 0.15 |
| P. trichocarpa | Plant | 664 | 144 | 215 | 392 | 103 | 202 | 0.40 |
| A. thaliana | Plant | 3,255 | 251 | 1,260 | 2,609 | 547 | 1,197 | 0.17 |
| O. sativa | Plant | 3,893 | 450 | 1,339 | 3,076 | 706 | 1,398 | 0.25 |
| P. patens | Plant | 2,068 | 249 | 534 | 1,156 | 558 | 727 | 0.32 |
| C. reinhardtii | Plant | 490 | 60 | 211 | 520 | 86 | 133 | 0.22 |
| O. lucimarinus | Plant | 17 | 0 | 13 | 60 | 2 | 2 | 0 |
| P. infestans | Protist | 406 | 6 | 262 | 564 | 60 | 78 | 0.02 |
| P. sojae | Protist | 66 | 1 | 32 | 87 | 15 | 18 | 0.03 |
| P. yoelii | Protist | 38 | 1 | 22 | 92 | 6 | 9 | 0.04 |
| P. falciparum | Protist | 77 | 9 | 34 | 75 | 19 | 15 | 0.21 |
| P. tetraurelia | Protist | 535 | 0 | 407 | 786 | 35 | 93 | 0 |
| T. thermophila | Protist | 282 | 6 | 202 | 423 | 30 | 44 | 0.03 |
| E. histolytica | Protist | 7 | 0 | 6 | 24 | 0 | 1 | 0 |
| P. tricornutum | Protist | 215 | 0 | 126 | 643 | 27 | 62 | 0 |
*For comparison, we include the total number of RIs predicted when unspliced ESTs are not discarded. Discarding unspliced ESTs primarily affects the number of predicted RIs. Complete results for all forms of alternative splicing, when unspliced ESTs are not discarded, are included in Additional data file 5.
All eukaryotes exhibit splice variation
We found that splice variation is present in all organisms we analyzed. Every eukaryote we studied exhibited RIs, and almost every organism exhibited competing 5' splice sites, competing 3' splice sites, and CEs. Several organisms showed zero or very few CEs or competing splice sites due to having only a small EST library or a small overall number of predicted splice variants (for example, Histoplasma capsulatum, Rhizopus oryzae, Entamoeba histolytica), or a small number of introns (for example, S. cerevisiae). We also found no CEs in Paramecium tetraurelia, despite a large EST library and numerous predicted splice variants. However, P. tetraurelia is unusual in that it has extraordinarily short introns (25 bp on average). As CEs are usually associated with longer introns and shorter exons, it is possible that this organism's gene structure renders CEs impossible.
Figure 2 illustrates the relative proportions of the four different kinds of splice variants in each organism, along with previously published data for human for comparison [8]. Our results for Caenorhabditis elegans, D. melanogaster, A. thaliana, and O. sativa confirmed those of previous studies [10,12,13].
Figure 2.
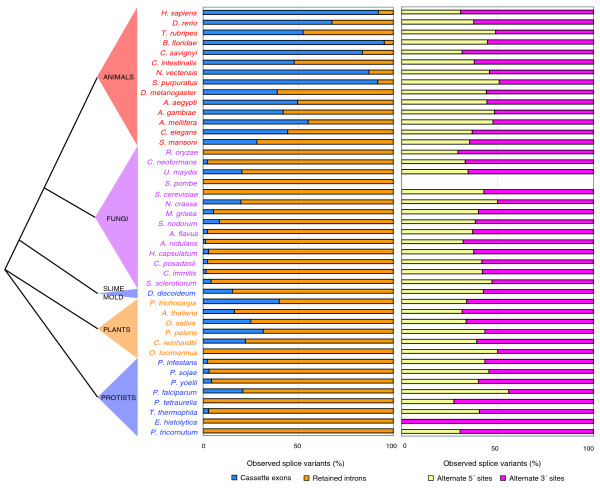
Frequencies of different forms of splice variation, arranged by phylogenetic group. The two bar charts show the relative frequencies of each type of splice variation. The ratio of CEs to RIs is shown in the chart on the left, while the one on the right displays competing 5' and competing 3' splice sites. Note that the CE/RI ratio shows wide variation among kingdoms while the ratio of competing 5' to competing 3' splice sites is remarkably consistent. A high-level overview of the phylogenetic tree is shown on the far left, and the organisms' names are colored according to their phylogenetic grouping. To see all four forms of splice variation on a single bar plot, see Additional data file 1. The data for H. sapiens was taken from a previous study [8].
The ratio of competing 3' splice sites to competing 5' splice sites was fairly constant, with more competing 3' splice sites than competing 5' splice sites in almost every case (Table 3). This is consistent with results of previous studies of splice variation, including the nine organisms in the Altextron database [10] and several other organisms [8,9,12]. When we combine the data from all the organisms in our analysis, there are 1.7 times more competing 3' splice sites than competing 5' splice sites. Interestingly, Zavolan et al. [26] found that competing 3' splice sites are more likely to preserve the reading frame than competing 5' splice sites.
In contrast to the uniform ratio of competing splice sites, the ratio of CEs to RIs varies widely between organisms. We found the ratio (which we will refer to as the CE fraction) to be a useful metric for summarizing the pattern of these splice variants. The CE fraction is listed in Table 3 and illustrated in Figure 2 for each organism.
CE and RI prevalence vary by kingdom and by intron length
Major eukaryotic groups (animals, plants, fungi, and protists) exhibit very divergent CE fractions (Figure 2). We found that RIs are the dominant form of splice variation in fungi and most protists, while CEs are the dominant form in multicellular animals. Plants have intermediate proportions of CEs and RIs. The difference in the proportions of RIs and CEs between the group of animals and the group consisting of all fungi and protists is highly statistically significant (p < 1e-10 by Fisher's exact test).
The 13 multicellular animals in our analysis have 1.3 times more CEs than RIs, which corresponds to an overall average CE fraction of 55%. CE fractions for these organisms range from 28% for the flatworm Schistosoma mansoni to 95% for the chordate Branchiostoma floridae. The four insects we studied have an average CE fraction of 44%. Moreover, variation in the CE fraction within chordates appears to be associated with genome size, serving as a partial control for phylogenetic effects. Takifugu rubripes has a more compact genome than the other two chordates in our analysis (Danio rerio and B. floridae), and has a correspondingly lower CE fraction (53%) than they do (D. rerio has a CE fraction of 68%; B. floridae, 95%).
In contrast, among the unicellular fungi and protists, we see very few CEs and an overwhelming preference for RIs. Overall, fungi and protists have 37 times more RIs than CEs (an average CE fraction of 3%). This preference for intron retention is consistent with previous reports on baker's yeast and fission yeast [17,27], although our kingdom-wide sampling indicates RI predominance is not limited to the highly derived yeasts. RIs also dominate in C. neoformans [15], a member of a group of intron-rich fungi, indicating that RI dominance in fungi is not coupled with intron density.
Plants in turn appear intermediate between animals and fungi in their relative amounts of CEs and RIs. We examined four multicellular plants: A. thaliana, O. sativa (rice), Populus trichocarpa (cottonwood), and Physcomitrella patens (a moss), as well as two unicellular green algae (Chlamydomonas. reinhardtii and Ostreococcus lucimarinus). Overall, we found 3.1 times more RIs than CEs, with an average CE fraction of 24%, consistent with previous studies in A. thaliana and O. sativa [11-13]. The unicellular algae C. reinhardtii has a CE fraction of 22%, which is closer to the values seen in multicellular plants than other unicellular organisms. However, it has a large genome size for a unicellular organism (118 Mb). In contrast, O. lucimarinus is a much simpler unicellular green algae with smaller genome size (13 Mb), minimal cellular organization and no CEs, a genome structure that is more like those of unicellular fungi and protists.
The observed variation in CE fraction closely parallels variation in intron length. Animals and plants have more long introns (introns greater than 200 bp) than do protists and fungi (Table 4). In Figure 3 we plot the fraction of constitutive introns greater than 200 bp versus the CE fraction, and demonstrate a direct correlation between the presence of long introns and high incidence of CEs (y = 0.84x + 0.00; R2 = 0.73). This correlation also holds within each kingdom (fungi, protists, plants, and animals), providing a phylogenetic control. As discussed below, this correlation is consistent with the hypothesis that splice site recognition differs within these groups.
Table 4.
Intron and exon lengths for controls and splice variants
| Kingdom | Intron density* | Genome assembly size (Mb)† | Average intron length‡ | Average RI length§¶ | Average intron length next to CE¥ | No. of CEs with unambiguous boundaries# | % introns >200 bp** | Average exon length¶†† | Average internal exon length¶‡‡ | Average CE length§§ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D. rerio | Animal | 7.2 | 1,547 | 2,940 | 130 | 3,485 | 903 | 71% | 180 | 132 | 113 |
| T. rubripes | Animal | 8.1 | 393 | 582 | 126 | 641 | 22 | 45% | 140 | 119 | 103 |
| B. floridae | Animal | 5.7 | 923 | 1,283 | 115 | 1,424 | 135 | 91% | 151 | 124 | 111 |
| C. savignyi | Animal | 9.1 | 164 | 710 | 87 | 595 | 20 | 82% | 148 | 130 | 123 |
| C. intestinalis | Animal | 7.4 | 117 | 457 | 152 | 544 | 327 | 76% | 181 | 143 | 122 |
| N. vectensis | Animal | 4.1 | 357 | 823 | 167 | 820 | 26 | 79% | 165 | 110 | 90 |
| S. purpuratus | Animal | 907 | 1,819 | 176 | 2,085 | 40 | 99% | 174 | 141 | 133 | |
| D. melanogaster | Animal | 3.9 | 120 | 829 | 90 | 1,632 | 218 | 25% | 279 | 246 | 167 |
| A. aegypti | Animal | 3.1 | 1,384 | 4,614 | 106 | 7,752 | 112 | 42% | 309 | 252 | 204 |
| A. gambiae | Animal | 3.2 | 278 | 1,154 | 110 | 2,905 | 83 | 26% | 286 | 236 | 181 |
| A. mellifera | Animal | 5.4 | 454 | 1,171 | 107 | 2,969 | 98 | 40% | 195 | 172 | 153 |
| C. elegans | Animal | 5.5 | 100 | 259 | 84 | 497 | 176 | 30% | 189 | 181 | 151 |
| S. mansoni | Animal | 4.8 | 381 | 2,222 | 42 | 2,387 | 127 | 77% | 179 | 167 | 130 |
| R. oryzae | Fungi | 2.3 | 46 | 58 | 66 | - | 0 | 1% | 201 | 161 | - |
| C. neoformans | Fungi | 5.4 | 19 | 64 | 67 | 95 | 8 | 5% | 228 | 179 | 56 |
| U. maydis | Fungi | 0.8 | 20 | 168 | 134 | 288 | 5 | 18% | 256 | 143 | 52 |
| S. pombe | Fungi | 1.3 | 13 | 108 | 58 | - | 0 | 14% | 309 | 102 | - |
| S. cerevisiae | Fungi | 0.1 | 12 | 241 | 244 | - | 0 | 49% | 596 | 46 | - |
| N. crassa | Fungi | 1.8 | 39 | 115 | 126 | 171 | 2 | 11% | 206 | 142 | 78 |
| M. grisea | Fungi | 1.7 | 42 | 109 | 110 | 103 | 5 | 7% | 238 | 160 | 64 |
| S. nodorum | Fungi | 1.7 | 37 | 70 | 82 | 88 | 2 | 3% | 257 | 178 | 17 |
| A. flavus | Fungi | 2.3 | 40 | 76 | 76 | 76 | 1 | 2% | 249 | 161 | 90 |
| A. nidulans | Fungi | 2.8 | 30 | 74 | 87 | 78 | 1 | 2% | 204 | 151 | 8 |
| H. capsulatum | Fungi | 2.5 | 33 | 112 | 114 | 114 | 1 | 8% | 227 | 155 | 25 |
| C. posadasii | Fungi | 2.2 | 27 | 80 | 87 | 137 | 10 | 2% | 395 | 289 | 81 |
| C. immitis | Fungi | 2.5 | 29 | 80 | 83 | 89 | 3 | 2% | 349 | 244 | 54 |
| S. sclerotiorum | Fungi | 1.8 | 38 | 84 | 98 | 71 | 7 | 5% | 316 | 213 | 56 |
| D. discoideum | Protist | 1.3 | 34 | 145 | 107 | 252 | 3 | 13% | 265 | 231 | 110 |
| P. trichocarpa | Plant | 3.1 | 308 | 431 | 140 | 637 | 80 | 50% | 204 | 125 | 103 |
| A. thaliana | Plant | 4.9 | 119 | 180 | 118 | 242 | 138 | 23% | 201 | 143 | 101 |
| O. sativa | Plant | 4.4 | 371 | 462 | 134 | 725 | 299 | 46% | 212 | 135 | 116 |
| P. patens | Plant | 3.8 | 480 | 295 | 188 | 394 | 188 | 57% | 220 | 137 | 113 |
| C. reinhardtii | Plant | 7 | 118 | 264 | 148 | 376 | 30 | 56% | 172 | 121 | 103 |
| O. lucimarinus | Plant | 0.5 | 13 | 157 | 97 | - | 0 | 28% | 437 | 135 | - |
| P. infestans | Protist | 2 | 229 | 76 | 78 | 88 | 5 | 1% | 214 | 146 | 92 |
| P. sojae | Protist | 86 | 87 | 107 | 75 | 1 | 2% | 213 | 147 | 111 | |
| P. yoelii | Protist | 1.2 | 23 | 179 | 151 | 157 | 1 | 25% | 229 | 127 | 18 |
| P. falciparum | Protist | 1.8 | 23 | 157 | 119 | 176 | 8 | 24% | 189 | 112 | 113 |
| P. tetraurelia | Protist | 2.5 | 72 | 25 | 26 | - | 0 | 0% | 246 | 233 | - |
| T. thermophila | Protist | 3.3 | 104 | 132 | 103 | 136 | 3 | 17% | 234 | 166 | 83 |
| E. histolytica | Protist | 23 | 72 | 62 | - | 0 | 2% | 296 | 168 | - | |
| P. tricornutum | Protist | 0.8 | 26 | 128 | 109 | - | 0 | 12% | 446 | 410 | - |
*(Number of introns in genome/Number of genes in genome), calculated from genome annotations. †The length of the assembly used in our analysis. ‡Calculated from constitutive introns in EST alignments. §Average length of RIs seen in our EST alignments. ¶See Table S2 in Additional data file 4 for average lengths based on genome annotations. ¥Average length of the two introns surrounding each predicted CE in our EST alignments. #Number of CEs considered for 'Average intron length next to CE' in the previous column and in Figure 5. This excludes those CEs where the introns next to the CEs do not have identical boundaries between transcripts with and without that CE. **Fraction of constitutive introns longer than 200 bp. ††Calculated from constitutive exons in our EST alignments. ‡‡Calculated from constitutive exons in our EST alignments (excluding exons that cannot be CEs, namely, the first and last exons in a gene, and exons in genes without introns). §§Average length of CEs seen in our EST alignments.
Figure 3.
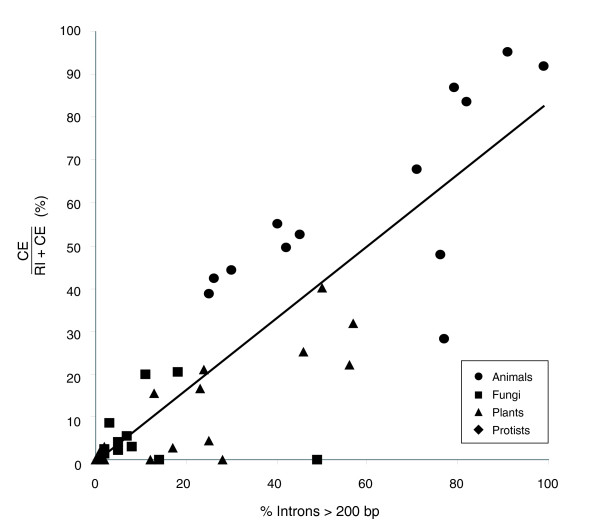
Relationship between long introns and CE fraction. The percentage of long introns (greater than 200 bp) is correlated with the CE fraction (). The best-fit line is y = 0.84x + 0.00 (R2 = 0.73). In each of four major eukaryotic groups (animals, fungi, plants and protists), species with more long introns display a higher propensity toward CEs.
Variably spliced regions exhibit size constraints
As shown in Table 4, variably spliced introns and exons are usually shorter than those in our data set that display no splice variation, in agreement with previous observations [28]. Moreover, these length differences between constitutive and variably spliced introns and exons appear to be associated with the relative frequencies of splice variation via CEs and RIs. In organisms where CEs are rare, such as fungi, CEs tend to be noticeably shorter than internal constitutive exons. However, in organisms with substantial fractions of CEs (animals and multicellular plants) we observe no significant length difference between CEs and internal constitutive exons (Figure 4). Intron retention displays the opposite behavior. In organisms where RIs are uncommon (animals and multicellular plants), RIs tend to be shorter than constitutive introns, while organisms with large numbers of RIs (fungi and protists) show no substantial length difference between RIs and constitutive introns (Figure 5). In general, CEs and RIs both tend to be shorter than their constitutively spliced counterparts, with the length difference most noticeable in organisms in which each splice variant was uncommon.
Figure 4.
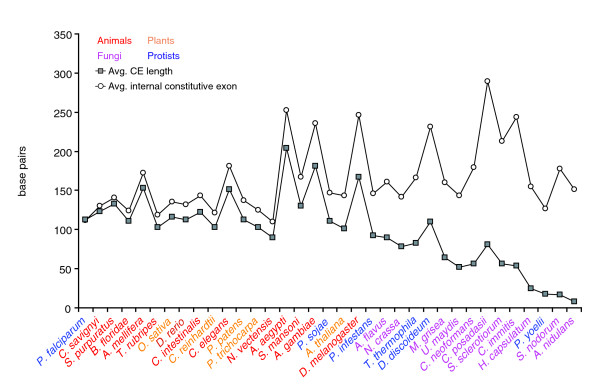
Average lengths of CEs compared to average lengths of internal constitutive exons. Species are sorted by the fractional difference between these two lengths. In organisms where CEs are common (animals and plants) CEs are almost identical in length to constitutive exons, while in species where CEs are rare (fungi and protists) CEs tend to be significantly shorter than constitutive exons. In animals and plants, where ED is common, CEs are spliced by the same process as constitutive exons and these two groups are thus subject to the same length constraints. In organisms that splice primarily by ID, including fungi and protists, the lengths of constitutive exons are not constrained by ED. However, CEs in these organisms are still recognized by ED. Thus, in these species, constitutive exons can grow longer than CEs.
Figure 5.
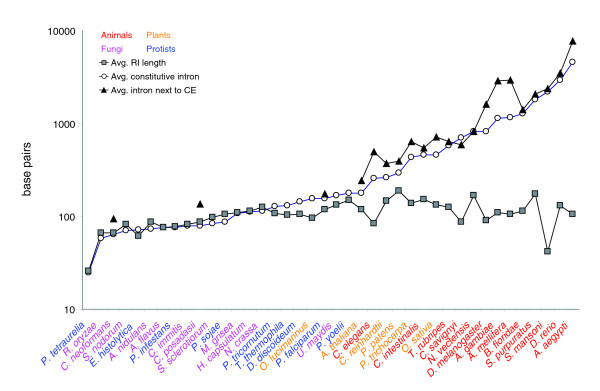
Average lengths of RIs compared with lengths of constitutive introns and introns adjacent to CEs. RI length, which is constrained by ID, is fairly constant across all organisms. In protists and fungi, average RI length is close to that of constitutive introns, because ID is the primary mode of splice site recognition for both groups. In animals, constitutive intron length differs substantially from RI length because most constitutive introns are recognized by ED and are not subject to the same length constraints as RIs, which are recognized by ID. Plants fall between unicellular organisms and animals. Data are shown only for organisms with at least five RIs. For introns next to CEs, data are shown only for organisms with at least eight CEs with unambiguous adjacent intron lengths on both sides. Introns next to CEs are usually longer than constitutive introns, because these introns are recognized by ED and are free from ID length constraints.
Most splice variants are not functional
We next sought to determine the degree to which the observed splice variants reflect programmed alternative splicing versus incomplete splicing or splicing errors. To do so, we examined the impact of observed splice variants on the corresponding open reading frame and resultant protein. We also examined conservation within regions containing splice variants to look for signatures of coding selection.
Previous analyses of splice variants in mammals have focused on the more prevalent CEs. One surprising result from these analyses is the high frequency of CEs that alter reading frame or introduce stop codons [29]. Overall, approximately half of human CEs in coding regions result in frameshifts, while an additional 15% of CEs that do not cause frameshifts introduce in-frame stop codons [29]. A more recent analysis of splice variants generated by the ENCODE consortium [30] revealed little evidence that alternative splice variants commonly give rise to functional isoforms. In the case of frameshifts, if both alternative open reading frames lead to functional proteins, one would expect the polymorphism or divergence level in all three codon positions to be the same [30]. Few splice variants in the ENCODE analysis displayed this property [30]. Our data are consistent with previous results. When looking at all 42 organisms in our analysis summed together (for a total of 7,115 CEs), CEs are more likely to have lengths that are a multiple of three (45% in all species examined), but over half of all CEs have lengths that leave remainders of one (28%) or two (27%) when divided by three.
Less has been reported about the functional impact of RIs. In humans, many RIs have been shown to be not merely partially spliced transcripts or splicing errors [31]. They were shown to have evidence of coding potential (having higher GC content than other introns, having codon usage more like exons, and having a lower frequency of stop codons). Many human RIs participate in coding for a protein domain (a smaller fraction than for exons, but a greater fraction than for constitutive introns) [31]. However, not all RIs in higher eukaryotes are necessarily functional. In plants, many RIs were shown to introduce premature termination codons or frameshifts [12].
The prevalence of RIs in all organisms we analyzed provides an opportunity to assess the possibility of a functional role for these observed events. Our analysis reveals that RIs do not display a preference for preserving reading frames: the lengths of all 11,925 observed RIs were roughly equally distributed between intron lengths evenly divisible by three (34%), and intron lengths with remainders of one (34%) and two (33%) when divided by three. Among the ten organisms with greater than 500 RIs, the number evenly divisible by three is 34 ± 2%, with a slightly higher value of 37% for D. rerio.
Though our analysis shows little evidence of frame preservation in RIs, we do see weak selection for coding potential. Between the closely related species of C. neoformans and Coccidioides immitis dN/dS ratios for concatenated RIs showed weak but significant evidence of conservation at the amino acid level (p < 0.001 for C. neoformans and p = 0.05 for C. immitis; Table 5). We also observe significantly fewer in-frame stop codons within RIs than in constitutive introns, with 23% fewer (p < 0.0001) in C. immitis, and 20% fewer (p = 0.02) in C. neoformans (Table 5). We observe no significant functional group over-representation (Table S3 in Additional data file 4).
Table 5.
dN/dS ratio and stop codon density for Ris
| dN/dS ratio for RIs | dN/dS for constitutive introns | p value | Stop codon density for RIs | Stop codon density for constitutive introns | p value | |
|---|---|---|---|---|---|---|
| C. neoformans | 0.8588 | 1.0008 | <0.001 | 0.0124 | 0.01534 | 0.02 |
| C. immitis | 0.7743 | 0.9083 | 0.049 | 0.01153 | 0.01507 | <0.0001 |
We thus see some evidence for coding potential in RIs, but taken together with previous observations of CEs, our results suggest that the majority of observed splice variants are unlikely to give rise to functional proteins. It has been proposed that splice variants leading to frameshifts or truncated proteins may be due in part to artifacts associated with EST library construction or sequencing. However, the universality of such disrupting variants across the many independent data sets and kingdoms analyzed here - and the occurrence of such disruptions associated with both RIs and CEs - suggest that these events occur naturally and frequently. In humans, plants, and fungi, transcripts containing premature stop codons are targeted for degradation through the process of nonsense mediated decay [32,33]. The widespread occurrence of premature stop codons in human splice variants has led to the hypothesis that unproductive splicing and translation may be pervasive [34]. Our results are consistent with this hypothesis.
Retained introns are associated with weak splice sites
Studies in mammals have demonstrated that splice sites adjacent to CEs and RIs are associated with weak splice site signals [28,35-40]. We evaluated RI information content in plants, fungi and protists and report results in agreement with previous studies.
We quantified 3' and 5' splice site strength by calculating the information content of the splice sites (see Materials and methods; Additional data file 2; Table S1 in Additional data file 4). We found significantly lower information content on either side of retained introns than constitutive introns for all 40 organisms in our analysis (P values 3.2e-9 and 2.1e-11, respectively, calculated from t-test). Overall, RI 5' splice sites had 1.9 ± 1.4 bits (24%) less information content, while their 3' splice sites had 0.9 ± 0.8 bits (17%) less (see data in Table S1 in Additional data file 4 and sequence logos [41] in Additional data file 2.) We observed the largest differences between RIs and constitutive introns in animals. The average differences in information content for 5' and 3' splice sites were, respectively, 2.3 and 1.0 bits for animals, 2.1 and 0.9 bits for fungi, 1.6 and 0.8 bits for protists, and 1.4 and 0.6 bits for plants.
While weak splice sites, such as those we observe in our RIs, have been associated with functional RIs and CEs [28,35-40], they are also expected to lead to greater occurrence of incomplete splicing [18]. Therefore, weaker splice sites are uninformative as to whether these RIs are functional or merely incomplete splicing.
Discussion
Splice variation reveals mechanisms of splice site recognition
The variation we observe in the CE fraction, the correspondence of this variation with average intron length, and the size constraints observed in both RIs and CEs can be parsimoniously explained by proposing differences in the proportion of splice junctions each organism recognizes via ED and ID. Organisms with a high CE fraction presumably splice predominantly via ED, whereas organisms with a low fraction presumably have a preference for ID-mediated splicing. Our results suggest that the fraction of introns recognized by ID and ED vary extensively across kingdoms, yet both mechanisms play a role in splicing in all phylogenetic groups.
In animals, exons are short relative to introns (Table 4) and CEs are more common than RIs (Table 3). This pattern is consistent with the hypothesis that splice junctions in animals are primarily recognized through ED, as has been previously demonstrated in vertebrates [19]. Moreover, the predominance of ED in these species predicts that CEs should be approximately as long as constitutive exons, because they are spliced the same way and, thus, are subject to the same length constraints. This is precisely the behavior we observe (Figure 4).
In fungi and protists, conversely, introns are short relative to exons (Table 4) and RIs far outnumber CEs. We propose that these groups primarily recognize splice junctions using ID (Figure 5). Because the splicing machinery in these organisms recognizes RIs in the same way it recognizes constitutive introns, we expect both types of intron should be subject to the same length constraints. Supporting this hypothesis, Figure 5 shows that constitutive introns in these species are similar in length to RIs. Intron definition has been demonstrated experimentally in the yeasts Saccharomyces and Schizosaccharomyces [17,27], and in plants [42]. Our analysis extends this result to all ascomycetes, as well as basidiomycetes and zygomycetes, and suggests that ID is not simply a characteristic of the derived yeasts. Furthermore, due to their high prevalence of RIs, we predict that ID predominates in the basidiomycete C. neoformans, which possesses 5.4 introns per gene on average, as well as the protozoan Tetrahymena thermophila, which has 3.3 introns per gene. ID is not associated with low intron density per se.
In plants, intron lengths vary widely, and individual species show substantial numbers of introns both greater and less than 200 bp in length (Table 4). Correspondingly, we observe that RIs and CEs both occur in sizeable quantities in this group. We thus propose that ID and ED both play significant roles in splice site recognition in plants.
While ED is most common in animals and ID dominates in fungi and protists, nearly all species analyzed show evidence for using both mechanisms. ID and ED have previously been shown to operate within the same species and indeed within the same gene [21]. We thus propose that the intron and exon length distributions in any organism are each sums of two distributions: one made up of shorter introns recognized by ID and the longer exons that surround them, and one made up of shorter exons recognized by ED and the surrounding longer introns. When we examine the lengths of CEs, we sample from only one of these two distributions: the subset of exons recognized by ED. When we examine the lengths of RIs, we sample from the length distribution of introns recognized by ID. Both these distributions are biased to be short, and this length bias is particularly noticeable in organisms where these splice variants are rare. Finally, when we look at the lengths of introns surrounding CEs, we are primarily sampling from the distribution of introns associated with ED. This last distribution tends to be long (Additional data file 3), as is the length distribution of exons surrounding RIs, which are associated with ID. (See Additional data file 3 for more detail on intron and exon length distributions in CEs and RIs.)
Importantly, our model of varying levels of ID or ED splice-site recognition explains the variation we see in CE fraction, whether or not individual variants lead to functional messages. As shown in Figure 1a, ID mis-recognition of a single splice site should lead to intron retention, as has been demonstrated by splice site mutation experiments in Drosophila and Schizosaccharomyces [17,18]. Creating a CE via ID, however, would theoretically require coordinated mis-recognition of two splice sites and pairing of splice sites over a greater distance. If this distance were greater than 200 bp, pairing with ID would be considerably hindered [24]. Similarly, as experimentally demonstrated and shown in Figure 1b, ED mis-recognition of a single splice site leads to a CE [19]. The hypothetical generation of RIs under ED would require multiple mis-recognitions and pairing of splice sites belonging to two possibly distant exons.
Thus, if many non-functional splice variants arise as a consequence of incorrect or incomplete splice site recognition, we would nonetheless expect that splice sites recognized by ID would more commonly give rise to RIs while those recognized by ED would more commonly give rise to CEs. Non-functional splice variants, therefore, are as informative as functional ones when considering the question of how splice sites are recognized. This is fortunate, as it is unclear at present what proportion of observed splice variation is indeed functionally significant. Splice variants and their characteristics, then, provide insight into the underlying mechanisms of splice site recognition, irrespective of whether such variants are functional or biological errors.
Evolutionary implications of splice variation
The varying prevalence of CEs and RIs across major eukaryotic groups raises evolutionary questions about modern variation in splice recognition among lineages, as well as the nature of splice site recognition in the last common eukaryotic ancestor. As RIs and CEs are exhibited in almost every organism we studied, it is likely that almost all extant eukaryotes are capable of recognizing splice sites via both ID and ED. The last common eukaryotic ancestor, then, may also have been capable of both types of splice site recognition. Indeed, Collins and Penny [43] report that most of the key components of the spliceosome were present in the last common eukaryotic ancestor. If the minimal mechanistic requirements to support both ID and ED were present in the last common eukaryotic ancestor, which was more prevalent: ID or ED? In our analysis, we note that intron density has less effect on the types of observed splice variation than does intron length. For example, the fungus C. neoformans recognizes splice sites almost exclusively by ID, despite having more introns per gene than many plants where ED is prevalent. Thus, whether the last common eukaryotic ancestor recognized splice sites predominantly via ID or ED is probably not a question of how many introns it had but rather how long its introns were. As very long introns appear to be a derived feature associated with multicellularity, we may speculate that the last eukaryotic common ancestor had introns similar in size to most protists, and, therefore, probably employed ID more than ED.
Evidence pertaining to the evolutionary origin of introns also suggests a prevalence of ID in early eukaryotes. Similarities in the splicing mechanism between self-splicing group II introns in prokaryotes and spliceosomal eukaryotic introns suggest that the former may have begat the latter [44-48]. As group II introns are mobile genetic elements that spread through retrotransposition, the ancestors of spliceosomal introns were probably self-contained in terms of their signals for excision, and would not likely have relied on splicing factors embedded in flanking coding sequence (as may be required for ED [49]). Therefore, the earliest spliceosomal introns may have employed a method of splice site recognition most similar to ID.
Recent molecular evidence suggests that SR (serine-arginine-rich) proteins may be associated with the ascendance of ED in animals and plants [50]. SR proteins bind to RNA sequences and assist in spliceosome assembly for both constitutively and alternatively spliced introns. They have been shown to enhance the splicing efficiency of introns with suboptimal splice signals in S. pombe [51]. Additionally, Shen and Green [52] have recently shown that SR proteins can rescue splicing of introns with suboptimal splice signals if those proteins are directed to bind to exonic mRNA sequence in S. cerevisiae, a species that has lost all native SR proteins. If the experimental binding of SR proteins to pre-mRNA sequences is indicative of the binding of SR proteins to exonic splicing enhancers in organisms where ED is prevalent, the fundamental splicing machinery of ID and ED may be closely related. Along these lines, Ram and Ast [50] speculate that the primary role of SR proteins has changed over time. In early eukaryotes, SR proteins assist in the recognition of suboptimal ID introns, while in higher eukaryotes, they bind to exonic splicing enhancers. This role change shifts the placement of the basal splicing machinery across exons instead of across introns and enables ED using the same spliceosomal machinery employed for ID [50].
The SR protein family has expanded in multicellular eukaryotic lineages [53], and this proliferation may have facilitated the widespread conversion of introns in those lineages from ID to ED. However, it is unclear what underlying neutral or selective forces could be responsible for this shift. Changes in genome size may have played a role. Multicellular eukaryotes generally exhibit larger genomes than unicellular eukaryotes, and organisms with large genomes tend to have long introns [54,55]. Because ID is only effective for introns less than approximately 250 bp [24], an upward trend in genome size in multicellular lineages (resulting from a reduced deletion rate, increased transposon activity, or both) could have favored ED introns that were spliceable in the face of this mutational pressure.
Regardless of which evolutionary forces are responsible for their ascendance, CEs produced through exon definition in multicellular eukaryotes allow for much greater flexibility and combinatorial complexity of alternatively spliced transcripts than would RIs recognized by ID. For example, the large number of transcripts (>30,000) that can be produced by the Dscam gene in D. melanogaster is facilitated by independent splicing of CEs [56]. The abundance of CEs enabled by ED in the human proteome may help to explain why the human genome contains only about as many genes as that of the worm or fly, despite the (admittedly biased) perception of our own much greater organismal complexity [57].
Conclusion
Using EST and cDNA data from 42 organisms, we find the prevalence of RIs and CEs to vary significantly across major eukaryotic groups, strongly suggesting that the underlying mode of splice site recognition (ID versus ED) also varies in prevalence across eukaryotes. Our results show that RIs, which are present in every organism we analyzed, are more widespread than previously thought. We also find a strong relationship between intron length and the prevalence of splice variation: the fraction of introns greater than 200 bp is correlated with the CE fraction. Shorter introns (<200 bp), such as those found in fungi and protists, are more likely to be recognized by ID. In all 23 fungi and protists that we examined, we observed that RIs are more common than CEs. In contrast, shorter exons surrounded by longer introns (>200-250 bp), such as those found in animals, are more likely to be recognized by ED. In the 13 multicellular animals in our analysis, CEs occur much more frequently, sometimes in greater numbers than RIs. The six plants in our analysis exhibited intermediate intron lengths, having more CEs than fungi and more RIs than animals.
We conclude that ID and ED are likely both present to some degree in all eukaryotes. We conclude that splicing proceeds primarily by ID in fungi and unicellular protists, due to the overwhelming majority of RIs and paucity of CEs observed in these organisms, as well as their short intron lengths and longer exon lengths. In contrast, splice sites in multicellular animals are recognized primarily via ED, due to the larger numbers of CEs observed, as well as these species' longer introns and shorter exons. However, the molecular mechanisms underlying the two different forms of recognition (ED and ID) are still unclear.
These findings help to reveal the complex interplay of selective constraints and mutational pressures underlying eukaryotic genome architecture, and improve our understanding of why eukaryotic genomes exhibit so much variation. Further sequencing of additional organisms, especially those that exhibit both unicellular and multicellular properties, will help to disentangle the effects of multicellularity, genome size, and intron length on the mechanisms of splice site recognition.
Materials and methods
EST alignments
All EST and assembly data were publicly available and downloaded from the Broad Institute [58], GenBank, J Craig Venter Institute/The Institute for Genomic Research [59], Joint Genome Institute [60] or VectorBase [61] (Tables 1 and 2). We used BLAT [62] version 33 to align the ESTs to genomic sequence using the following parameters: --minIdentity = 95 --minScore = 50 --queryType = rna. We set the --maxIntron parameter to the longest annotated intron in each species.
We filtered the resulting alignments using the following criteria: each alignment must contain at least one canonical splice site (GT:AG, CG:AG, AT:AC); must have no non-canonical splice sites; must have ≥ 95% nucleotide identity; must not have more than nine consecutive insertions; and must not have more than nine consecutive deletions outside of an intron. We also required three or more exact matches at every intron-exon boundary [12]. If a single EST aligned to more than one location on the genome, we only considered the alignment with the highest score. To guard against redundant input data, if multiple alignments in the same locus had the same sequence after trimming, we disregarded all but one of them.
We discarded all unspliced alignments to prevent labeling pre-spliced transcripts as splice variants. Unspliced ESTs show no evidence of having been processed by the spliceosome, and may represent pre-spliced transcript fragments. It is difficult to distinguish between an unspliced EST that has been processed and an unspliced EST that has not, and thus we cannot report how many processed ESTs we discarded. However, if most unspliced ESTs have already been processed by the spliceosome, we would expect to find more of them in organisms with very few introns and/or very long exons. We found no such correlation in either case (Additional data file 5). We conclude that a substantial fraction of the unspliced ESTs represent pre-spliced ESTs, and, therefore, that unspliced ESTs are not a reliable indicator of splice variation.
The number of RIs changed substantially depending on whether we included or excluded unspliced ESTs, while the numbers of CEs and competing 3' and 5' splice sites changed very little. We believe that previous reports that do not exclude unspliced ESTs overestimate the frequency of intron retention.
After aligning the ESTs and filtering them, we built transcripts and transcript fragments using CallReferenceGenes, an unpublished tool used in the Broad Institute's genome annotation pipeline since 2005. Several previous papers provide an in-depth discussion of the problem [12,63]. We briefly sketch our algorithm here. The source code for CallReferenceGenes, as well as the code that labels alternative splice forms, is included in Additional data file 6.
First, we partition the alignments into clusters, so that every alignment has exon-exon overlap with at least one other alignment in the cluster, and no alignment has exon-exon overlap with any alignment outside its cluster. Alignments that overlap no other alignments are ignored.
Next, for each cluster, we compare every alignment to every other alignment that overlaps it. (Here, as opposed to the previous step, we also consider exon-intron overlaps.) Relationships are directional, and are given one of three labels: 'conflicts', 'extended-by', or 'includes'. Two alignments conflict if any base in the region of overlap is exonic in one alignment and intronic in the other. If they do not conflict, one alignment extends another if it ends after the other and does not begin before it. Lastly, one alignment includes another if it does not conflict with it, starts before it, and ends after it.
At this stage the cluster is represented by a graph of nodes (representing alignments) and labeled edges (representing their relationships). To turn this into a more tree-like representation that is easier to traverse, we build an ordered list of alignments. We sort them by 3' coordinate, in ascending order. In the case where we have two alignments with identical 3' coordinates, we sort by 5' coordinate, again in ascending order. In this way, no alignment is extended by any element that sorts before it. An alignment usually, though not always, sorts after the alignments it includes.
To improve performance, we prune redundant relationships from the graph. We apply a series of heuristics to reduce the number of paths from an alignment to its descendants. These 'trimming rules' include: first, if A is extended by B, and C extends both A and B, and there is no element D such that B conflicts with D and C does not, then any path containing A and B will also contain C. The extended-by relationship from A to C is therefore redundant. Second, if A is included by B, and there is no element D such that B conflicts with D and A does not, then any path containing A will also include B. All extended-by relationships terminating in A are redundant. Third, if A is extended by B and both are included by C, and every element D that conflicts with C also conflicts with either A or B, then any path containing both A and B will also contain C. Thus, the extended-by relationship from A to B is redundant.
We traverse the list bottom-up so that every element is pruned before any element it extends. There will be multiple paths from a parent to a given child if a splice variation lies between them. If no splice variation occurs between them, generally, there will be one path, but the above rules are not exhaustive and some duplicate paths may remain after pruning. As we traverse the list and prune it, we track, per alignment, all alignments that can be reached through it (for example, all its extenders and includees, as well as their extenders and includees, and so on). We call these sets of alignments the 'descendants' of each alignment. (Note that pruning never reduces the membership of these sets, only the number of edges between them.)
After ordering and pruning, we can traverse the list of alignments left-to-right, following 'extended-by' and 'includes' links to build paths linking splice-compatible alignments. To do this, we do the following. First, find starts - a start is any element that extends no other element and has at least one descendant that cannot be reached through any previously discovered start. Second, walk the tree - treating each start, in turn, as a root of a subtree, traverse extension and inclusion links as edges in a graph; each unique path represents (a fragment of) a transcript. Third, remove sub-paths - if all the alignments in one path are present in another, we discard the one containing fewer alignments. Fourth, overlapping paths with distinct alignments represent splice variants of the same gene.
Given two overlapping transcripts A and B, all bases in the region of overlap will be in one of four states: I, A and B are exonic; II, A is exonic; III, B is exonic; IV, neither is exonic. We group all adjacent states into a column with a single label, then use a sliding-window approach, across three such columns, to compare overlapping transcripts. CEs, RIs, and competing 5' and 3' splice sites all have a different signature appearance. CEs appear as IV:II:IV and IV:III:IV; RIs appear as I:II:I and I:III:I; competing 5' and 3' splice sites appear as I:II:IV, I:III:IV, IV:II:I, and IV:III:I.
We filter the set of splice variants by requiring that every base in the variant region be exonic in at least one alignment and intronic in at least one alignment. Because EST data are fragmentary we cannot be sure that initial or terminal exons are complete. To ensure accurate labeling as well as reliable length statistics, we require that any exon in the alternatively spliced region not be an initial or terminal exon. Finally, we require that every alignment spanning the region conform to either the major or minor variant. The reported length of each splice variant is simply the width of the center column in the windows described above.
We also generated a list of constitutive introns and exons as a control. These are introns and exons predicted from loci where no ESTs conflict. Furthermore, every base within these constitutive elements must have ten or more ESTs supporting it. Note that there is no way to tell for sure that any exon or intron never exhibits splice variation; this method simply identifies loci where splice variation has never been seen, and if present, is presumably rare.
Testing retained introns for evidence of selection at the codon level
We used a comparative approach to test retained introns for purifying selection at the codon level using two groups of fungi, where genome sequences at suitable evolutionary distances were available. We examined all four sequenced serotypes of C. neoformans (JEC21, H99, R265 and WM276) in one group, while the second group consisted of C. immitis and the C735 strain of Coccidioides posadasii. Orthology of genes was determined using a reciprocal-best-BLAST criterion, while the alignment of orthologs was performed using ClustalW [64]. Alignments of retained orthologous introns were concatenated to enhance power for detecting selection. Prior to concatenation, splice donor and acceptor sites were removed, and the 5' and 3' ends of each intron alignment were padded with gaps in order to preserve the native reading frames of introns. We used a 100 bp cutoff for retained introns in C. neoformans and 150 bp in C. immitis. Since 95% of the introns we identified in each organism were less than these cutoffs, we used a cutoff to eliminate unusually long introns. We analyzed 477 and 389 orthologous intron alignments in C. neoformans and C. immitis, respectively. The dN/dS ratio of concatenated intron alignments was calculated using the codeml program (model M0) in the PAML 3.15 software package [65]. The probability that the resulting dN/dS ratios were significantly less than one, indicating purifying selection at the codon level, was calculated using a bootstrapping approach with a set of control introns. We identified 672 and 662 control introns in C. neoformans and C. immitis, respectively. We randomly resampled, with replacement, from the control introns to create 1,000 concatenated control alignments approximately equal in length to the concatenated retained intron alignments in each taxonomic group. Then, we used codeml to calculate the dN/dS ratio exhibited by each resampled control alignment to determine the probability of observing dN/dS ratios as low as or lower than those exhibited by the retained introns in each taxonomic group, under a null hypothesis that they are non-coding.
Abbreviations
CE, cassette exon; ED, exon definition; EST, expressed sequence tag; ID, intron definition; RI, retained intron; SR, serine-arginine-rich.
Authors' contributions
AMM and MDP contributed equally. MDP wrote the EST alignment software and contributed to writing the paper. AMM performed the analysis and drafted and finalized the manuscript. DEN performed the dN/dS and stop codon density analyses, and contributed to writing the paper. JEG initiated and supervised the study, and revised the manuscript.
Additional data files
The following additional data are available with the online version of this paper. Additional data file 1 is a figure showing the frequencies of different forms of splice variation. Additional file 2 is a figure showing sequence logos for splice sites for both RIs and controls. Additional data file 3 shows intron and exon length distributions for six example organisms. Additional data file 4 contains supplementary tables detailing the differences in information content between RIs and controls (Table S1), intron and exon lengths from annotations (Table S2), and details of our analysis of functional group enrichment of RIs (Table S3). Additional data file 5 is a spreadsheet containing a comparison of alternative splicing events for the situations where unspliced ESTs are included as well as discarded. This spreadsheet also includes data for a higher-confidence dataset requiring a greater number of ESTs supporting each predicted alternative splicing event. Additional data file 6 contains computer source codes for the CallReferenceGenes program, as well as the code that labels alternative splice forms. These codes can be inspected but will not be functional without the rest of the Broad Institute's Calhoun environment.
Supplementary Material
The data for H. sapiens were taken from a previous study [8].
(a) Sequence logos [41] for 3' splice sites of RIs and control introns. (b) Sequence logos [41] for 5' splice sites of RIs and control introns. For 5' splice sites, we show sequence logos for six intronic and three exonic bases. For 3' splice sites, we show sequence logos for three intronic and one exonic bases.
Six example organisms with large numbers of splice variants were chosen and their normalized intron and exon length distributions are plotted here. (a) CE distributions are shifted towards shorter lengths than the control exon length distribution, because of constraints on exon length imposed by ED. (b) The peak at short intron lengths is very similar in RIs and constitutive introns, because this short intron peak is primarily made up of introns recognized by ID. In contrast, almost no introns surrounding CEs have lengths close to this 'intron-definition peak' - almost all of them are spread out over a wide range of longer intron lengths, and have low values in the area of the short intron-length ID peak shown here (0-200 bp).
Table S1: differences in information content between RIs and controls. Table S2: intron and exon lengths from annotations. Table S3: details of our analysis of functional group enrichment of RIs.
A comparison of alternative splicing events for the situations where unspliced ESTs are included as well as discarded. We also include data for a higher-confidence dataset requiring a greater number of ESTs supporting each predicted alternative splicing event.
We include the source code for the CallReferenceGenes program, as well as the code which labels alternative splice forms. These codes can be inspected but will not be functional without the rest of the Broad Institute's Calhoun environment. These are included as Unix tar files.
Contributor Information
Abigail M McGuire, Email: amcguire@broad.mit.edu.
Matthew D Pearson, Email: mdp@broad.mit.edu.
Daniel E Neafsey, Email: neafsey@broad.mit.edu.
James E Galagan, Email: jgalag@mit.edu.
Acknowledgements
Preliminary sequence data for S. mansoni and H. capsulatum were obtained from the website of The Institute for Genomic Research/The J Craig Venter Institute. Sequencing of S. mansoni is supported by award from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The genome sequencing of H. capsulatum was supported by the National Institute of Allergy and Infectious Diseases (NIAID). The sequence data for B. floridae, C. reinhardtii, P. tricornutum, and P. patens were produced by the US Department of Energy Joint Genome Institute. The sequences of C. savignyi, C. immitis, H. capsulatum, P. infestans, R. oryzae, S. sclerotiorum, and S. nodorum were produced by the Broad Institute; EST data for C. posadasii not generated at JCVI and ESTs from C. immitis were produced by the Coccidioides Genome Resources Consortium (CGRC) as part of the Broad's Comparative Coccidioides Genome Project. EST data for S. sclerotiorum were produced by the Broad Institute (Christina Cuomo) as part of a grant with Christina Cuomo, Marty Dickman, Jeffrey Rollins, and Linda Kohn. S. sclerotiorum EST libraries were constructed by Jeffrey Rollins, Dave Edwards, Adrienne Sexton, and Barbara Howlett. The sequence data for D. rerio were produced by the D. rerio Sequencing Group at the Sanger Institute. We would like to thank Michael Koehrsen and his engineering team for their development of the Calhoun bioinformatics platform, without which this large-scale analysis would have been impossible. We would also like to thank the anonymous reviewers, who made many helpful suggestions to improve this paper. This research was funded by an NSF grant for Comparative Fungal Genomics (MCB-0450812), and an NIAID Contract for Microbial Genome Centers (HHSN26620040001C).
References
- Roy SW, Gilbert W. The evolution of spliceosomal introns: patterns, puzzles and progress. Nat Rev Genet. 2006;7:211–221. doi: 10.1038/nrg1807. [DOI] [PubMed] [Google Scholar]
- Jeffares DC, Mourier T, Penny D. The biology of intron gain and loss. Trends Genet. 2006;22:16–22. doi: 10.1016/j.tig.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Hirschman JE, Balakrishnan R, Christie KR, Costanzo MC, Dwight SS, Engel SR, Fisk DG, Hong EL, Livstone MS, Nash R, Park J, Oughtred R, Skrzypek M, Starr B, Theesfeld CL, Williams J, Andrada R, Binkley G, Dong Q, Lane C, Miyasato S, Sethuraman A, Schroeder M, Thanawala MK, Weng S, Dolinski K, Botstein D, Cherry JM. Genome Snapshot: a new resource at the Saccharomyces Genome Database (SGD) presenting an overview of the Saccharomyces cerevisiae genome. Nucleic Acids Res. 2006. pp. D442–D445. [DOI] [PMC free article] [PubMed]
- Loftus B, Anderson I, Davies R, Alsmark UC, Samuelson J, Amedeo P, Roncaglia P, Berriman M, Hirt RP, Mann BJ, Nozaki T, Suh B, Pop M, Duchene M, Ackers J, Tannich E, Leippe M, Hofer M, Bruchhaus I, Willhoeft U, Bhattacharya A, Chillingworth T, Churcher C, Hance Z, Harris B, Harris D, Jagels K, Moule S, Mungall K, Ormond D. et al. The genome of the protist parasite Entamoeba histolytica. Nature. 2005;433:865–868. doi: 10.1038/nature03291. [DOI] [PubMed] [Google Scholar]
- Blencowe BJ. Alternative splicing: new insights from global analyses. Cell. 2006;126:37–47. doi: 10.1016/j.cell.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Matlin AJ, Clark F, Smith CW. Understanding alternative splicing: towards a cellular code. Nat Rev Mol Cell Biol. 2005;6:386–398. doi: 10.1038/nrm1645. [DOI] [PubMed] [Google Scholar]
- Kwan T, Benovoy D, Dias C, Gurd S, Serre D, Zuzan H, Clark TA, Schweitzer A, Staples MK, Wang H, Blume JE, Hudson TJ, Sladek R, Majewski J. Heritability of alternative splicing in the human genome. Genome Res. 2007;17:1210–1218. doi: 10.1101/gr.6281007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugnet CW, Kent WJ, Ares M, Jr, Haussler D. Transcriptome and genome conservation of alternative splicing events in humans and mice. Pac Symp Biocomput. 2004. pp. 66–77. [DOI] [PubMed]
- Ast G. How did alternative splicing evolve? Nat Rev Genet. 2004;5:773–782. doi: 10.1038/nrg1451. [DOI] [PubMed] [Google Scholar]
- Thanaraj TA, Stamm S, Clark F, Riethoven JJ, Le Texier V, Muilu J. ASD: the Alternative Splicing Database. Nucleic Acids Res. 2004. pp. D64–D69. [DOI] [PMC free article] [PubMed]
- Ner-Gaon H, Halachmi R, Savaldi-Goldstein S, Rubin E, Ophir R, Fluhr R. Intron retention is a major phenomenon in alternative splicing in Arabidopsis. Plant J. 2004;39:877–885. doi: 10.1111/j.1365-313X.2004.02172.x. [DOI] [PubMed] [Google Scholar]
- Campbell MA, Haas BJ, Hamilton JP, Mount SM, Buell CR. Comprehensive analysis of alternative splicing in rice and comparative analyses with Arabidopsis. BMC Genomics. 2006;7:327. doi: 10.1186/1471-2164-7-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BB, Brendel V. Genomewide comparative analysis of alternative splicing in plants. Proc Natl Acad Sci USA. 2006;103:7175–7180. doi: 10.1073/pnas.0602039103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DM, Drabenstot SD, Buchanan KL, Lai H, Zhu H, Dyer DW, Roe BA, Murphy JW. Introns and splicing elements of five diverse fungi. Eukaryot Cell. 2004;3:1088–1100. doi: 10.1128/EC.3.5.1088-1100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, Bruno D, Vamathevan J, Miranda M, Anderson IJ, Fraser JA, Allen JE, Bosdet IE, Brent MR, Chiu R, Doering TL, Donlin MJ, D'Souza CA, Fox DS, Grinberg V, Fu J, Fukushima M, Haas BJ, Huang JC, Janbon G, Jones SJ, Koo HL, Krzywinski MI, Kwon-Chung JK, Lengeler KB, Maiti R. et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science. 2005;307:1321–1324. doi: 10.1126/science.1103773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L, Penny D. Proceedings of the SMBE Tri-National Young Investigators' Workshop 2005. Investigating the intron recognition mechanism in eukaryotes. Mol Biol Evol. 2006;23:901–910. doi: 10.1093/molbev/msj084. [DOI] [PubMed] [Google Scholar]
- Romfo CM, Alvarez CJ, van Heeckeren WJ, Webb CJ, Wise JA. Evidence for splice site pairing via intron definition in Schizosaccharomyces pombe. Mol Cell Biol. 2000;20:7955–7970. doi: 10.1128/MCB.20.21.7955-7970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talerico M, Berget SM. Intron definition in splicing of small Drosophila introns. Mol Cell Biol. 1994;14:3434–3445. doi: 10.1128/mcb.14.5.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talerico M, Berget SM. Effect of 5' splice site mutations on splicing of the preceding intron. Mol Cell Biol. 1990;10:6299–6305. doi: 10.1128/mcb.10.12.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget SM. Exon recognition in vertebrate splicing. J Biol Chem. 1995;270:2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- Kennedy CF, Kramer A, Berget SM. A role for SRp54 during intron bridging of small introns with pyrimidine tracts upstream of the branch point. Mol Cell Biol. 1998;18:5425–5434. doi: 10.1128/mcb.18.9.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson BL, Cote GJ, Berget SM. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990;10:84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LP, Burge CB. A computational analysis of sequence features involved in recognition of short introns. Proc Natl Acad Sci USA. 2001;98:11193–11198. doi: 10.1073/pnas.201407298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox-Walsh KL, Dou Y, Lam BJ, Hung SP, Baldi PF, Hertel KJ. The architecture of pre-mRNAs affects mechanisms of splice-site pairing. Proc Natl Acad Sci USA. 2005;102:16176–16181. doi: 10.1073/pnas.0508489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locations of predicted sites of splice variation. http://www.broad.mit.edu/ftp/pub/seq/msc/pub/altsplice_data.tar.gz http://www.broad.mit.edu/ftp/pub/seq/msc/pub/altsplice_data.tar.gz
- Zavolan M, Kondo S, Schonbach C, Adachi J, Hume DA, Hayashizaki Y, Gaasterland T. Impact of alternative initiation, splicing, and termination on the diversity of the mRNA transcripts encoded by the mouse transcriptome. Genome Res. 2003;13:1290–1300. doi: 10.1101/gr.1017303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe KJ, Ares M Jr. Intron self-complementarity enforces exon inclusion in a yeast pre-mRNA. Proc Natl Acad Sci USA. 1997;94:12467–12472. doi: 10.1073/pnas.94.23.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm S, Zhu J, Nakai K, Stoilov P, Stoss O, Zhang MQ. An alternative-exon database and its statistical analysis. DNA Cell Biol. 2000;19:739–756. doi: 10.1089/104454900750058107. [DOI] [PubMed] [Google Scholar]
- Sorek R, Shamir R, Ast G. How prevalent is functional alternative splicing in the human genome? Trends Genet. 2004;20:68–71. doi: 10.1016/j.tig.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Tress ML, Martelli PL, Frankish A, Reeves GA, Wesselink JJ, Yeats C, Olason PL, Albrecht M, Hegyi H, Giorgetti A, Raimondo D, Lagarde J, Laskowski RA, López G, Sadowski MI, Watson JD, Fariselli P, Rossi I, Nagy A, Kai W, Størling Z, Orsini M, Assenov Y, Blankenburg H, Huthmacher C, Ramírez F, Schlicker A, Denoeud F, Jones P, Kerrien S. et al. The implications of alternative splicing in the ENCODE protein complement. Proc Natl Acad Sci USA. 2007;104:5495–5500. doi: 10.1073/pnas.0700800104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante PA, Sakabe NJ, Kirschbaum-Slager N, de Souza SJ. Detection and evaluation of intron retention events in the human transcriptome. Rna. 2004;10:757–765. doi: 10.1261/rna.5123504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu-Yilik G, Gehring NH, Hentze MW, Kulozik AE. Nonsense-mediated mRNA decay: from vacuum cleaner to Swiss army knife. Genome Biol. 2004;5:218. doi: 10.1186/gb-2004-5-4-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YF, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem. 2007;76:51–74. doi: 10.1146/annurev.biochem.76.050106.093909. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Green RE, Brenner SE. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc Natl Acad Sci USA. 2003;100:189–192. doi: 10.1073/pnas.0136770100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakabe NJ, de Souza SJ. Sequence features responsible for intron retention in human. BMC Genomics. 2007;8:59. doi: 10.1186/1471-2164-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza I, Schellenberg GD. tau Exon 10 expression involves a bipartite intron 10 regulatory sequence and weak 5' and 3' splice sites. J Biol Chem. 2002;277:26587–26599. doi: 10.1074/jbc.M203794200. [DOI] [PubMed] [Google Scholar]
- Dye BT, Buvoli M, Mayer SA, Lin CH, Patton JG. Enhancer elements activate the weak 3' splice site of alpha-tropomyosin exon 2. Rna. 1998;4:1523–1536. doi: 10.1017/S1355838298980360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigueur A, La Branche H, Kornblihtt AR, Chabot B. A splicing enhancer in the human fibronectin alternate ED1 exon interacts with SR proteins and stimulates U2 snRNP binding. Genes Dev. 1993;7:2405–2417. doi: 10.1101/gad.7.12a.2405. [DOI] [PubMed] [Google Scholar]
- Clark F, Thanaraj TA. Categorization and characterization of transcript-confirmed constitutively and alternatively spliced introns and exons from human. Hum Mol Genet. 2002;11:451–464. doi: 10.1093/hmg/11.4.451. [DOI] [PubMed] [Google Scholar]
- Garg K, Green P. Differing patterns of selection in alternative and constitutive splice sites. Genome Res. 2007;17:1015–1022. doi: 10.1101/gr.6347907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider TD, Stephens RM. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AJ, Baynton CE, Schuler MA. Interactions across exons can influence splice site recognition in plant nuclei. Plant Cell. 1996;8:2295–2307. doi: 10.1105/tpc.8.12.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L, Penny D. Complex spliceosomal organization ancestral to extant eukaryotes. Mol Biol Evol. 2005;22:1053–1066. doi: 10.1093/molbev/msi091. [DOI] [PubMed] [Google Scholar]
- Cech TR. The generality of self-splicing RNA: relationship to nuclear mRNA splicing. Cell. 1986;44:207–210. doi: 10.1016/0092-8674(86)90751-8. [DOI] [PubMed] [Google Scholar]
- Rogers JH. How were introns inserted into nuclear genes? Trends Genet. 1989;5:213–216. doi: 10.1016/0168-9525(89)90084-X. [DOI] [PubMed] [Google Scholar]
- Sharp PA. "Five easy pieces". Science. 1991;254:663. doi: 10.1126/science.1948046. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Intron phylogeny: a new hypothesis. Trends Genet. 1991;7:145–148. [PubMed] [Google Scholar]
- Stoltzfus A. On the possibility of constructive neutral evolution. J Mol Evol. 1999;49:169–181. doi: 10.1007/PL00006540. [DOI] [PubMed] [Google Scholar]
- Blencowe BJ. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem Sci. 2000;25:106–110. doi: 10.1016/S0968-0004(00)01549-8. [DOI] [PubMed] [Google Scholar]
- Ram O, Ast G. SR proteins: a foot on the exon before the transition from intron to exon definition. Trends Genet. 2007;23:5–7. doi: 10.1016/j.tig.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Webb CJ, Romfo CM, van Heeckeren WJ, Wise JA. Exonic splicing enhancers in fission yeast: functional conservation demonstrates an early evolutionary origin. Genes Dev. 2005;19:242–254. doi: 10.1101/gad.1265905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Green MR. RS domains contact splicing signals and promote splicing by a common mechanism in yeast through humans. Genes Dev. 2006;20:1755–1765. doi: 10.1101/gad.1422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Morais NL, Carmo-Fonseca M, Aparicio S. Systematic genome-wide annotation of spliceosomal proteins reveals differential gene family expansion. Genome Res. 2006;16:66–77. doi: 10.1101/gr.3936206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov AE. Intron-genome size relationship on a large evolutionary scale. J Mol Evol. 1999;49:376–384. doi: 10.1007/PL00006561. [DOI] [PubMed] [Google Scholar]
- Petrov DA. Mutational equilibrium model of genome size evolution. Theor Popul Biol. 2002;61:531–544. doi: 10.1006/tpbi.2002.1605. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–684. doi: 10.1016/S0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C. et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- The Broad Institute of Harvard and MIT. http://www.broad.mit.edu http://www.broad.mit.edu
- The Institute for Genomic Research: The J Craig Venter Institute. http://www.tigr.org/ http://www.tigr.org/
- US Department of Energy Joint Genome Institute. http://www.jgi.doe.gov/ http://www.jgi.doe.gov/
- Vectorbase. http://www.vectorbase.org http://www.vectorbase.org
- Kent WJ. BLAT - the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. Article published online before March 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyras E, Caccamo M, Curwen V, Clamp M. ESTGenes: alternative splicing from ESTs in Ensembl. Genome Res. 2004;14:976–987. doi: 10.1101/gr.1862204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- Danio rerio Sequencing Group at the Sanger Institute. http://www.sanger.ac.uk/Projects/D_rerio/ http://www.sanger.ac.uk/Projects/D_rerio/
- Aparicio S, Chapman J, Stupka E, Putnam N, Chia JM, Dehal P, Christoffels A, Rash S, Hoon S, Smit A, Gelpke MD, Roach J, Oh T, Ho IY, Wong M, Detter C, Verhoef F, Predki P, Tay A, Lucas S, Richardson P, Smith SF, Clark MS, Edwards YJ, Doggett N, Zharkikh A, Tavtigian SV, Pruss D, Barnstead M, Evans C. et al. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science. 2002;297:1301–1310. doi: 10.1126/science.1072104. [DOI] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein DM, Harafuji N, Hastings KE, Ho I, Hotta K, Huang W, Kawashima T, Lemaire P, Martinez D, Meinertzhagen IA, Necula S, Nonaka M, Putnam N, Rash S, Saiga H, Satake M, Terry A, Yamada L, Wang HG, Awazu S, Azumi K. et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- Sea Urchin Genome Sequencing Consortium. Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, Angerer RC, Angerer LM, Arnone MI, Burgess DR, Burke RD, Coffman JA, Dean M, Elphick MR, Ettensohn CA, Foltz KR, Hamdoun A, Hynes RO, Klein WH, Marzluff W, McClay DR, Morris RL, Mushegian A, Rast JP, Smith LC, Thorndyke MC, Vacquier VD, Wessel GM, Wray G, Zhang L. et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR. et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M. et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D, Arensburger P, Atkinson P, Besansky NJ, Bruggner RV, Butler R, Campbell KS, Christophides GK, Christley S, Dialynas E, Emmert D, Hammond M, Hill CA, Kennedy RC, Lobo NF, MacCallum MR, Madey G, Megy K, Redmond S, Russo S, Severson DW, Stinson EO, Topalis P, Zdobnov EM, Birney E, Gelbart WM, Kafatos FC, Louis C, Collins FH. VectorBase: a home for invertebrate vectors of human pathogens. Nucleic Acids Res. 2007. pp. D503–D505. [DOI] [PMC free article] [PubMed]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M. et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. elegans, Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Kämper J, Kahmann R, Bölker M, Ma LJ, Brefort T, Saville BJ, Banuett F, Kronstad JW, Gold SE, Müller O, Perlin MH, Wösten HA, de Vries R, Ruiz-Herrera J, Reynaga-Peña CG, Snetselaar K, McCann M, Pérez-Martín J, Feldbrügge M, Basse CW, Steinberg G, Ibeas JI, Holloman W, Guzman P, Farman M, Stajich JE, Sentandreu R, González-Prieto JM, Kennell JC, Molina L. et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature. 2006;444:97–101. doi: 10.1038/nature05248. [DOI] [PubMed] [Google Scholar]
- Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, Stewart A, Sgouros J, Peat N, Hayles J, Baker S, Basham D, Bowman S, Brooks K, Brown D, Brown S, Chillingworth T, Churcher C, Collins M, Connor R, Cronin A, Davis P, Feltwell T, Fraser A, Gentles S, Goble A, Hamlin N, Harris D, Hidalgo J, Hodgson G, Holroyd S. et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–880. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, Feldmann H, Galibert F, Hoheisel JD, Jacq C, Johnston M, Louis EJ, Mewes HW, Murakami Y, Philippsen P, Tettelin H, Oliver SG. Life with 6000 genes. Science. 1996;274:563–547. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Borkovich KA, Selker EU, Read ND, Jaffe D, FitzHugh W, Ma LJ, Smirnov S, Purcell S, Rehman B, Elkins T, Engels R, Wang S, Nielsen CB, Butler J, Endrizzi M, Qui D, Ianakiev P, Bell-Pedersen D, Nelson MA, Werner-Washburne M, Selitrennikoff CP, Kinsey JA, Braun EL, Zelter A, Schulte U, Kothe GO, Jedd G, Mewes W. et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422:859–868. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu JR, Pan H, Read ND, Lee YH, Carbone I, Brown D, Oh YY, Donofrio N, Jeong JS, Soanes DM, Djonovic S, Kolomiets E, Rehmeyer C, Li W, Harding M, Kim S, Lebrun MH, Bohnert H, Coughlan S, Butler J, Calvo S, Ma LJ. et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434:980–986. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Cuomo C, Ma LJ, Wortman JR, Batzoglou S, Lee SI, Basturkmen M, Spevak CC, Clutterbuck J, Kapitonov V, Jurka J, Scazzocchio C, Farman M, Butler J, Purcell S, Harris S, Braus GH, Draht O, Busch S, D'Enfert C, Bouchier C, Goldman GH, Bell-Pedersen D, Griffiths-Jones S, Doonan JH, Yu J, Vienken K, Pain A, Freitag M. et al. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 2005;438:1105–1115. doi: 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- Eichinger L, Pachebat JA, Glöckner G, Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski K, Xu Q, Tunggal B, Kummerfeld S, Madera M, Konfortov BA, Rivero F, Bankier AT, Lehmann R, Hamlin N, Davies R, Gaudet P, Fey P, Pilcher K, Chen G, Saunders D, Sodergren E, Davis P, Kerhornou A, Nie X, Hall N, Anjard C. et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A, Schein J, Sterck L, Aerts A, Bhalerao RR, Bhalerao RP, Blaudez D, Boerjan W, Brun A, Brunner A, Busov V, Campbell M, Carlson J, Chalot M, Chapman J, Chen GL, Cooper D, Coutinho PM, Couturier J, Covert S, Cronk Q. et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, Hadley D, Hutchison D, Martin C, Katagiri F, Lange BM, Moughamer T, Xia Y, Budworth P, Zhong J, Miguel T, Paszkowski U, Zhang S, Colbert M, Sun WL, Chen L, Cooper B, Park S, Wood TC, Mao L, Quail P. et al. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science. 2002;296:92–100. doi: 10.1126/science.1068275. [DOI] [PubMed] [Google Scholar]
- Palenik B, Grimwood J, Aerts A, Rouzé P, Salamov A, Putnam N, Dupont C, Jorgensen R, Derelle E, Rombauts S, Zhou K, Otillar R, Merchant SS, Podell S, Gaasterland T, Napoli C, Gendler K, Manuell A, Tai V, Vallon O, Piganeau G, Jancek S, Heijde M, Jabbari K, Bowler C, Lohr M, Robbens S, Werner G, Dubchak I, Pazour GJ. et al. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc Natl Acad Sci USA. 2007;104:7705–7710. doi: 10.1073/pnas.0611046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler BM, Tripathy S, Zhang X, Dehal P, Jiang RH, Aerts A, Arredondo FD, Baxter L, Bensasson D, Beynon JL, Chapman J, Damasceno CM, Dorrance AE, Dou D, Dickerman AW, Dubchak IL, Garbelotto M, Gijzen M, Gordon SG, Govers F, Grunwald NJ, Huang W, Ivors KL, Jones RW, Kamoun S, Krampis K, Lamour KH, Lee MK, McDonald WH, Medina M. et al. Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science. 2006;313:1261–1266. doi: 10.1126/science.1128796. [DOI] [PubMed] [Google Scholar]
- Carlton JM, Angiuoli SV, Suh BB, Kooij TW, Pertea M, Silva JC, Ermolaeva MD, Allen JE, Selengut JD, Koo HL, Peterson JD, Pop M, Kosack DS, Shumway MF, Bidwell SL, Shallom SJ, van Aken SE, Riedmuller SB, Feldblyum TV, Cho JK, Quackenbush J, Sedegah M, Shoaibi A, Cummings LM, Florens L, Yates JR, Raine JD, Sinden RE, Harris MA, Cunningham DA. et al. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature. 2002;419:512–519. doi: 10.1038/nature01099. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM. et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aury JM, Jaillon O, Duret L, Noel B, Jubin C, Porcel BM, Ségurens B, Daubin V, Anthouard V, Aiach N, Arnaiz O, Billaut A, Beisson J, Blanc I, Bouhouche K, Câmara F, Duharcourt S, Guigo R, Gogendeau D, Katinka M, Keller AM, Kissmehl R, Klotz C, Koll F, Le Mouël A, Lepère G, Malinsky S, Nowacki M, Nowak JK, Plattner H. et al. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature. 2006;444:171–178. doi: 10.1038/nature05230. [DOI] [PubMed] [Google Scholar]
- Eisen JA, Coyne RS, Wu M, Wu D, Thiagarajan M, Wortman JR, Badger JH, Ren Q, Amedeo P, Jones KM, Tallon LJ, Delcher AL, Salzberg SL, Silva JC, Haas BJ, Majoros WH, Farzad M, Carlton JM, Smith RK Jr, Garg J, Pearlman RE, Karrer KM, Sun L, Manning G, Elde NC, Turkewitz AP, Asai DJ, Wilkes DE, Wang Y, Cai H. et al. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 2006;4:e286. doi: 10.1371/journal.pbio.0040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The data for H. sapiens were taken from a previous study [8].
(a) Sequence logos [41] for 3' splice sites of RIs and control introns. (b) Sequence logos [41] for 5' splice sites of RIs and control introns. For 5' splice sites, we show sequence logos for six intronic and three exonic bases. For 3' splice sites, we show sequence logos for three intronic and one exonic bases.
Six example organisms with large numbers of splice variants were chosen and their normalized intron and exon length distributions are plotted here. (a) CE distributions are shifted towards shorter lengths than the control exon length distribution, because of constraints on exon length imposed by ED. (b) The peak at short intron lengths is very similar in RIs and constitutive introns, because this short intron peak is primarily made up of introns recognized by ID. In contrast, almost no introns surrounding CEs have lengths close to this 'intron-definition peak' - almost all of them are spread out over a wide range of longer intron lengths, and have low values in the area of the short intron-length ID peak shown here (0-200 bp).
Table S1: differences in information content between RIs and controls. Table S2: intron and exon lengths from annotations. Table S3: details of our analysis of functional group enrichment of RIs.
A comparison of alternative splicing events for the situations where unspliced ESTs are included as well as discarded. We also include data for a higher-confidence dataset requiring a greater number of ESTs supporting each predicted alternative splicing event.
We include the source code for the CallReferenceGenes program, as well as the code which labels alternative splice forms. These codes can be inspected but will not be functional without the rest of the Broad Institute's Calhoun environment. These are included as Unix tar files.


