Abstract
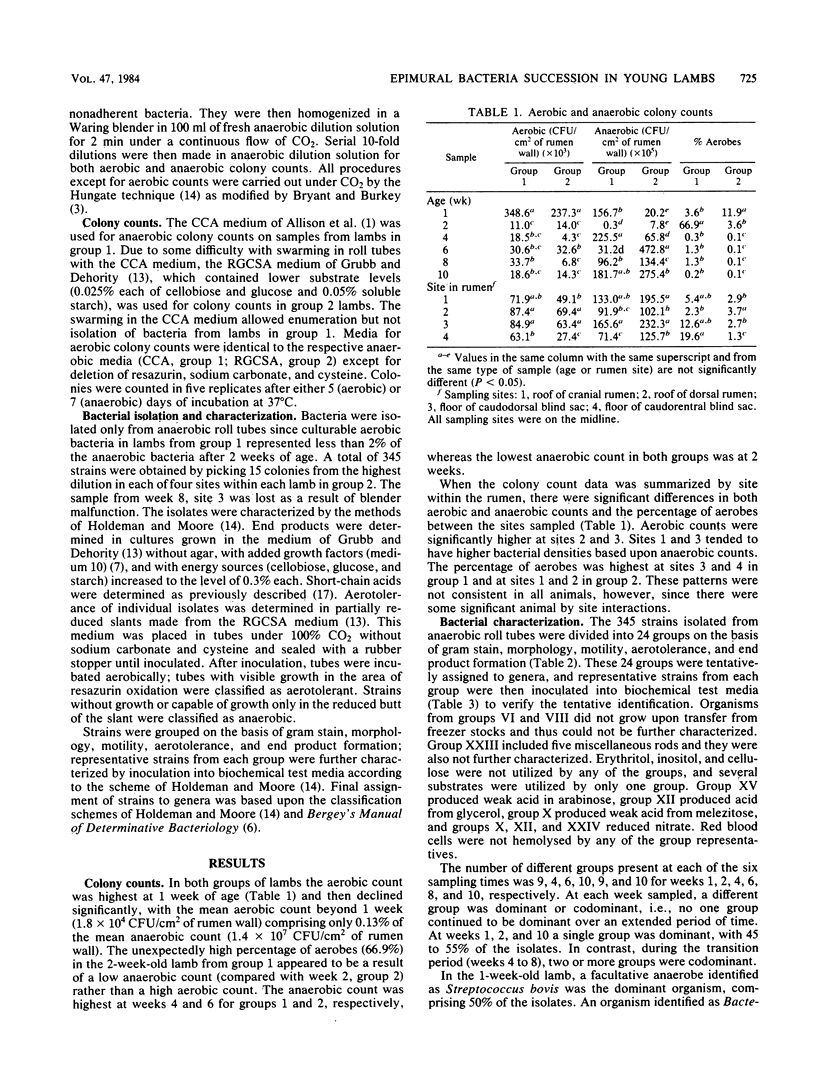
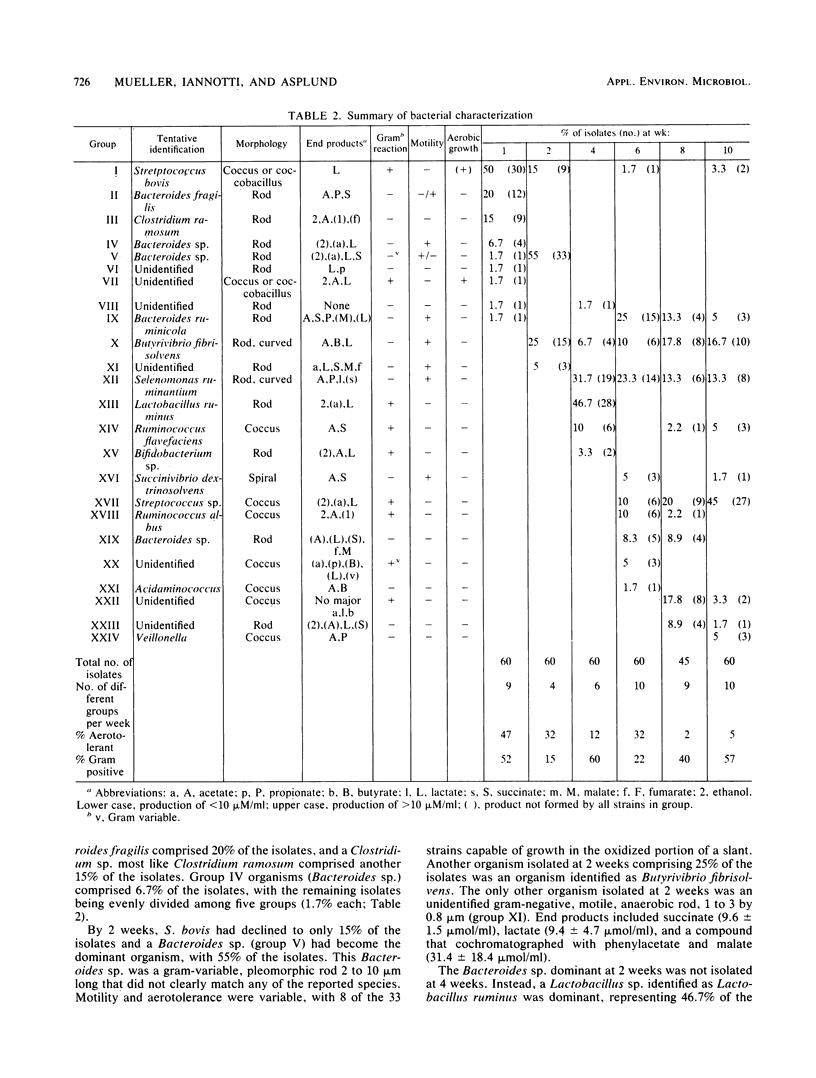
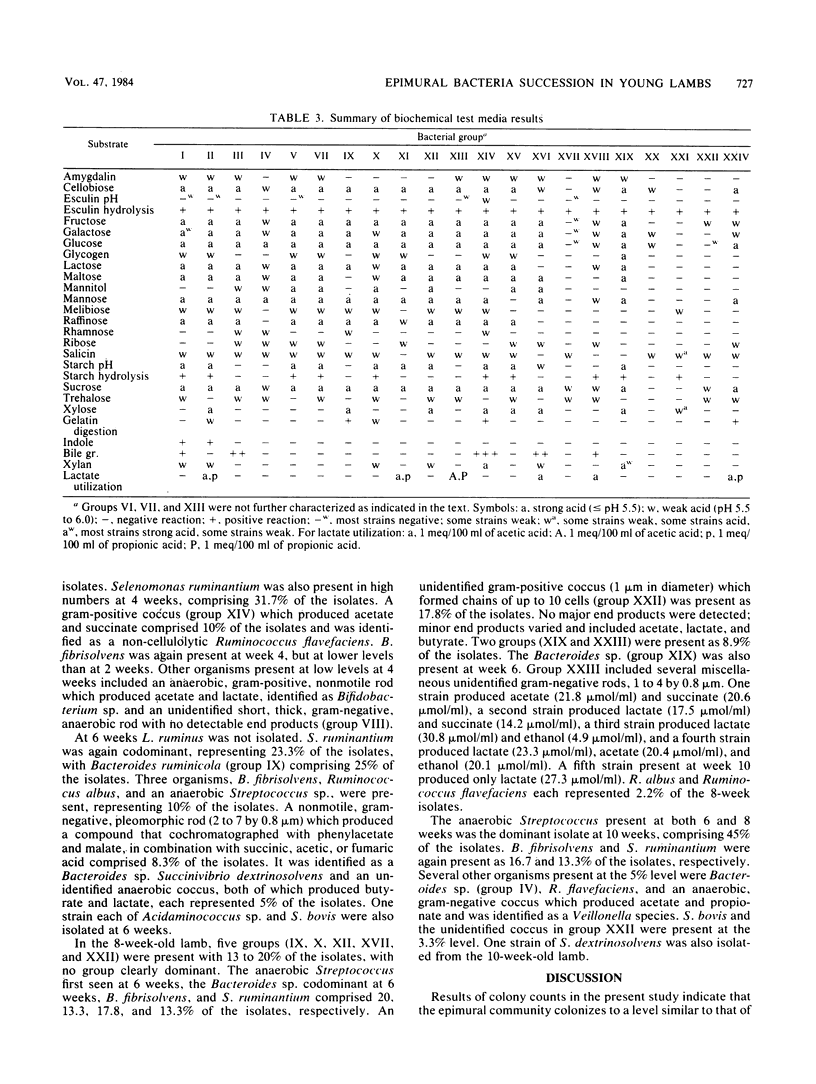
Successive changes in aerobic and anaerobic bacterial counts and changes in the generic composition of the epimural community in lambs from 1 to 10 weeks were determined. Bacterial culture counts revealed a predominantly anaerobic community, with the mean anaerobic count being 1.4 X 10(7) CFU/cm2 of tissue surface. The aerobic count was highest at 1 week of age and declined significantly thereafter to a mean of 1.8 X 10(4) CFU/cm2, thus representing only 0.13% of the mean anaerobic count after week 1. Of the 345 strains isolated anaerobically at 1, 2, 4, 6, 8, and 10 weeks of age, 47, 32, 12, 32, 2, and 5% were capable of growth in a partially reduced medium, indicating a reduction in the number of facultative anaerobes with time. The majority of isolated strains were identified as belonging to genera commonly isolated from rumen contents. In some instances, however, strains did not correspond to previously described species, and some genera were present in proportions different from those expected in rumen fluid. At three of the sampling times, one genus was dominant, constituting 45 to 55% of the isolates. These dominant isolates were Streptococcus bovis, Bacteroides sp., and an anaerobic Streptococcus sp. for weeks 1, 2, and 10, respectively. During the transition period (weeks 4 to 8), two or more groups were codominant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. J., Robinson I. M., Bucklin J. A., Booth G. D. Comparison of bacterial populations of the pig cecum and colon based upon enumeration with specific energy sources. Appl Environ Microbiol. 1979 Jun;37(6):1142–1151. doi: 10.1128/aem.37.6.1142-1151.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T., Clarke R. T., Newhook J. C. Scanning electron microscope study of bacteria associated with the rumen epithelium of sheep. Appl Microbiol. 1975 Oct;30(4):668–675. doi: 10.1128/am.30.4.668-675.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., McCowan R. P., Costerton J. W. Adherent epithelial bacteria in ruminants and their roles in digestive tract function. Am J Clin Nutr. 1979 Jan;32(1):139–148. doi: 10.1093/ajcn/32.1.139. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Wallace R. J. The mechanism of passage of endogenous urea through the rumen wall and the role of ureolytic epithelial bacteria in the urea flux. Br J Nutr. 1979 Nov;42(3):553–557. doi: 10.1079/bjn19790147. [DOI] [PubMed] [Google Scholar]
- Dehority B. A., Grubb J. A. Bacterial population adherent to the epithelium on the roo of the dorsal rumen of sheep. Appl Environ Microbiol. 1981 Jun;41(6):1424–1427. doi: 10.1128/aem.41.6.1424-1427.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsdale D., Cheng K. J., Wallace R. J., Goodlad R. A. Digestion of epithelial tissue of the rumen wall by adherent bacteria in infused and conventionally fed sheep. Appl Environ Microbiol. 1980 May;39(5):1059–1066. doi: 10.1128/aem.39.5.1059-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb J. A., Dehority B. A. Variation in colony counts of total viable anaerobic rumen bacteria as influenced by media and cultural methods. Appl Environ Microbiol. 1976 Feb;31(2):262–267. doi: 10.1128/aem.31.2.262-267.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy P. M., Clarke R. T., Milligan L. P. Influences of dietary sucrose and urea on transfer of endogenous urea to the rumen of sheep and numbers of epithelial bacteria. Br J Nutr. 1981 Nov;46(3):533–541. doi: 10.1079/bjn19810062. [DOI] [PubMed] [Google Scholar]
- Mead L. J., Jones G. A. Isolation and presumptive identification of adherent epithelial bacteria ("epimural" bacteria) from the ovine rumen wall. Appl Environ Microbiol. 1981 Apr;41(4):1020–1028. doi: 10.1128/aem.41.4.1020-1028.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R. E., Asplund J. M., Iannotti E. L. Successive changes in the epimural bacterial community of young lambs as revealed by scanning electron microscopy. Appl Environ Microbiol. 1984 Apr;47(4):715–723. doi: 10.1128/aem.47.4.715-723.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamate H., Kikuchi T., Onodera A., Nagatani T. Scanning electron microscopic observation on the surface structure of the bovine rumen mucosa. Arch Histol Jpn. 1971 Oct;33(4):273–282. doi: 10.1679/aohc1950.33.273. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Cheng K. J., Dinsdale D., Orskov E. R. An independent microbial flora of the epithelium and its role in the ecomicrobiology of the rumen. Nature. 1979 May 31;279(5712):424–426. doi: 10.1038/279424a0. [DOI] [PubMed] [Google Scholar]


