Abstract
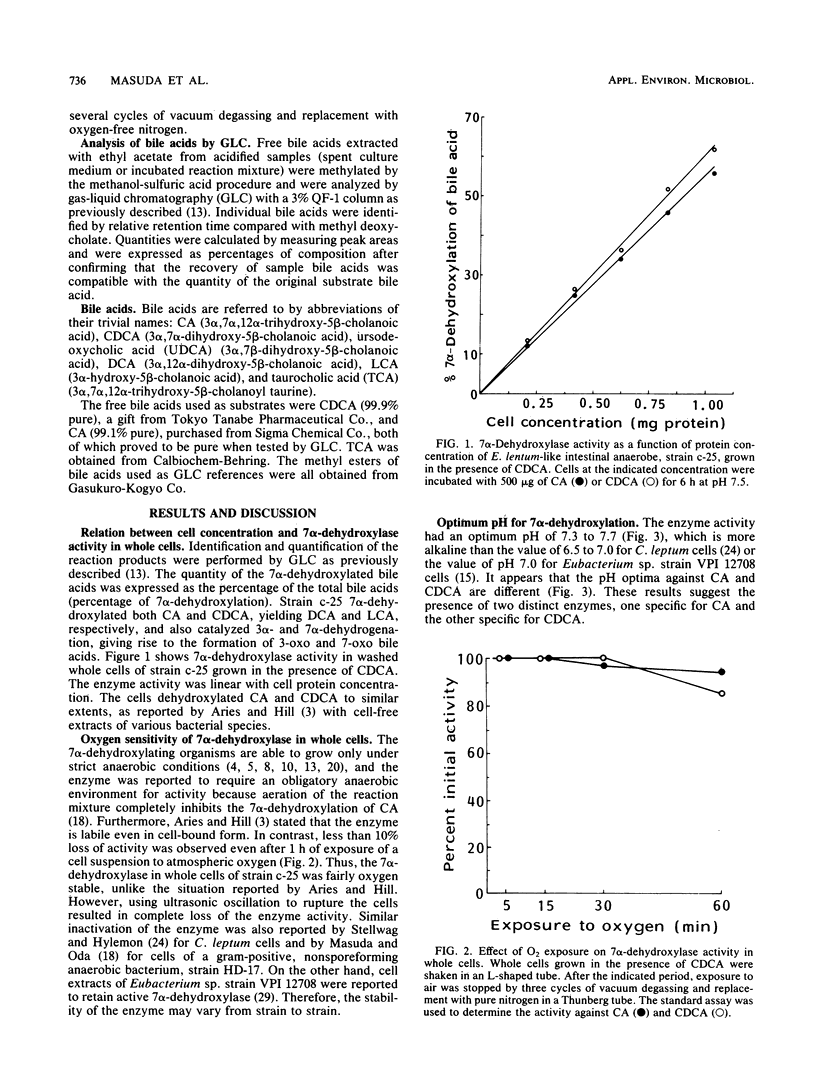
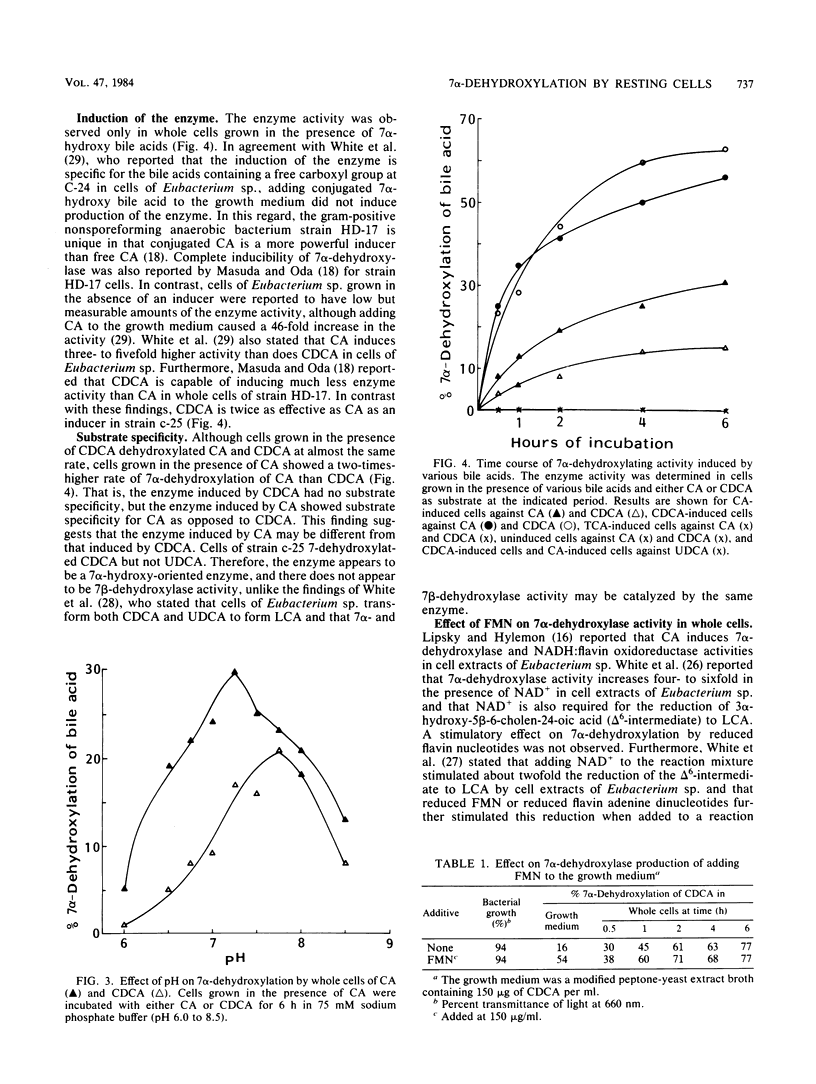
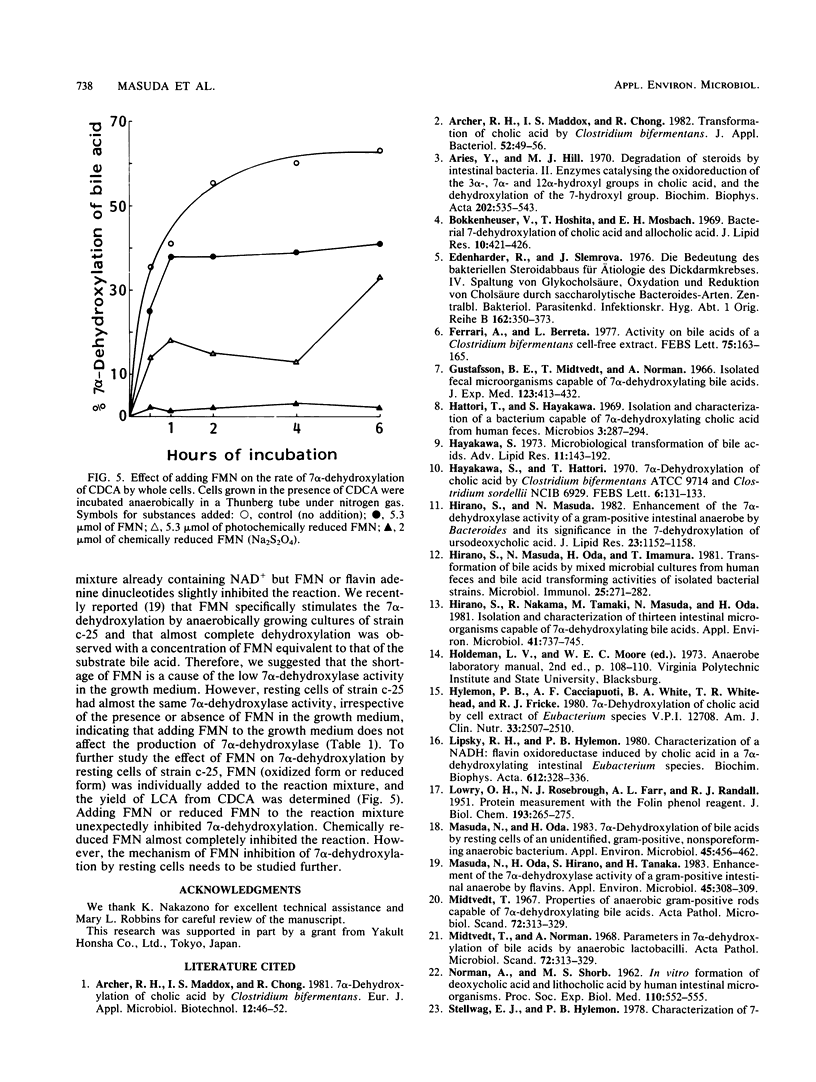
7 alpha-Dehydroxylation of cholic acid and chenodeoxycholic acid by whole cells of strain c-25, a Eubacterium lentum-like intestinal anaerobe, was studied. 7 alpha-Dehydroxylase activity was observed only in whole cells grown in the presence of the primary bile acid (cholic acid or chenodeoxycholic acid). Chenodeoxycholic acid was twice as effective as cholic acid as an inducer. Although cells grown in the presence of chenodeoxycholic acid had no significant substrate specificity for the two primary bile acids, cells grown in the presence of cholic acid showed two times greater activity against cholic acid than chenodeoxycholic acid. Exposure of cell suspensions to atmospheric oxygen resulted in little loss of the 7 alpha-dehydroxylase activity. The induced enzyme had an optimal pH range of 7.3 to 7.7. Although adding flavin mononucleotide to the growth medium significantly increased the 7 alpha-dehydroxylation of bile acids without an increase in cell growth, inhibition of the enzyme activity was observed in the resting cell system when flavin mononucleotide was included in the reaction mixture.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aries V., Hill M. J. Degradation of steroids by intestinal bacteria. II. Enzymes catalysing the oxidoreduction of the 3 alpha-, 7 alpha- and 12 alpha-hydroxyl groups in cholic acid, and the dehydroxylation of the 7-hydroxyl group. Biochim Biophys Acta. 1970 May 5;202(3):535–543. doi: 10.1016/0005-2760(70)90124-4. [DOI] [PubMed] [Google Scholar]
- Bokkenheuser V., Hoshita T., Mosbach E. H. Bacterial 7-dehydroxylation of cholic acid and allocholic acid. J Lipid Res. 1969 Jul;10(4):421–426. [PubMed] [Google Scholar]
- Edenharder R., Slemrova J. Die Bedeutung des bakteriellen Steroidabbaus für die Atiologie des Dickdarmkrebses. IV. Spaltung von Glykocholsäure, Oxydation und Reduktion von Cholsäure durch saccharolytische Bacteroides-Arten. Zentralbl Bakteriol Orig B. 1976 Jul;162(3-4):350–373. [PubMed] [Google Scholar]
- Ferrari A., Beretta L. Activity on bile acids of a Clostridium bifermentans cell-free extract. FEBS Lett. 1977 Mar 15;75(1):163–165. doi: 10.1016/0014-5793(77)80076-8. [DOI] [PubMed] [Google Scholar]
- Gustafsson B. E., Midtvedt T., Norman A. Isolated fecal microorganisms capable of 7-alpha-dehydroxylating bile acids. J Exp Med. 1966 Feb 1;123(2):413–432. doi: 10.1084/jem.123.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa S., Hattori T. 7alpha-dehydroxylation of cholic acid by Clostridium bifermentans strain ATCC 9714 and Clostridium sordellii strain NCIB 6929. FEBS Lett. 1970 Jan 26;6(2):131–133. doi: 10.1016/0014-5793(70)80020-5. [DOI] [PubMed] [Google Scholar]
- Hayakawa S. Microbiological transformation of bile acids. Adv Lipid Res. 1973;11:143–192. doi: 10.1016/b978-0-12-024911-4.50011-8. [DOI] [PubMed] [Google Scholar]
- Hirano S., Masuda N. Enhancement of the 7 alpha-dehydroxylase activity of a gram-positive intestinal anaerobe by Bacteroides and its significance in the 7-dehydroxylation of ursodeoxycholic acid. J Lipid Res. 1982 Nov;23(8):1152–1158. [PubMed] [Google Scholar]
- Hirano S., Masuda N., Oda H., Imamura T. Transformation of bile acids by mixed microbial cultures from human feces and bile acid transforming activities of isolated bacterial strains. Microbiol Immunol. 1981;25(3):271–282. doi: 10.1111/j.1348-0421.1981.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Hirano S., Nakama R., Tamaki M., Masuda N., Oda H. Isolation and characterization of thirteen intestinal microorganisms capable of 7 alpha-dehydroxylating bile acids. Appl Environ Microbiol. 1981 Mar;41(3):737–745. doi: 10.1128/aem.41.3.737-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Cacciapuoti A. F., White B. A., Whitehead T. R., Fricke R. J. 7 alpha-Dehydroxylation of cholic acid by cell extracts of Eubacterium species V.P.I. 12708. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2507–2510. doi: 10.1093/ajcn/33.11.2507. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipsky R. H., Hylemon P. B. Characterization of a NADH:flavin oxidoreductase induced by cholic acid in a 7 alpha-dehydroxylating intestinal Eubacterium species. Biochim Biophys Acta. 1980 Apr 11;612(2):328–336. doi: 10.1016/0005-2744(80)90115-1. [DOI] [PubMed] [Google Scholar]
- Masuda N., Oda H. 7 alpha-Dehydroxylation of bile acids by resting cells of an unidentified, gram-positive, nonsporeforming anaerobic bacterium. Appl Environ Microbiol. 1983 Feb;45(2):456–462. doi: 10.1128/aem.45.2.456-462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N., Oda H., Hirano S., Tanaka H. Enhancement of the 7 alpha-dehydroxylase activity of a gram-positive intestinal anaerobe by flavins. Appl Environ Microbiol. 1983 Jan;45(1):308–309. doi: 10.1128/aem.45.1.308-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midtvedt T., Norman A. Parameters in 7-alpha-dehydroxylation of bile acids by anaerobic lactobacilli. Acta Pathol Microbiol Scand. 1968;72(2):313–329. doi: 10.1111/j.1699-0463.1968.tb01345.x. [DOI] [PubMed] [Google Scholar]
- NORMAN A., SHORB M. S. In vitro formation of deoxycholic and lithocholic acid by human intestinal microorganisms. Proc Soc Exp Biol Med. 1962 Jul;110:552–555. doi: 10.3181/00379727-110-27577. [DOI] [PubMed] [Google Scholar]
- Stellwag E. J., Hylemon P. B. 7alpha-Dehydroxylation of cholic acid and chenodeoxycholic acid by Clostridium leptum. J Lipid Res. 1979 Mar;20(3):325–333. [PubMed] [Google Scholar]
- Stellwag E. J., Hylemon P. B. Characterization of 7-alpha-dehydroxylase in Clostridium leptum. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S243–S247. doi: 10.1093/ajcn/31.10.S243. [DOI] [PubMed] [Google Scholar]
- White B. A., Cacciapuoti A. F., Fricke R. J., Whitehead T. R., Mosbach E. H., Hylemon P. B. Cofactor requiremets for 7 alpha-dehydroxylation of cholic and chenodeoxycholic acid in cell extracts of the intestinal anaerobic bacterium, Eubacterium species V.P.I. 13708. J Lipid Res. 1981 Aug;22(6):891–898. [PubMed] [Google Scholar]
- White B. A., Fricke R. J., Hylemon P. B. 7 beta-Dehydroxylation of ursodeoxycholic acid by whole cells and cell extracts of the intestinal anaerobic bacterium, Eubacterium species V.P.I. 12708. J Lipid Res. 1982 Jan;23(1):145–153. [PubMed] [Google Scholar]
- White B. A., Lipsky R. L., Fricke R. J., Hylemon P. B. Bile acid induction specificity of 7 alpha-dehydroxylase activity in an intestinal Eubacterium species. Steroids. 1980 Jan;35(1):103–109. doi: 10.1016/0039-128x(80)90115-4. [DOI] [PubMed] [Google Scholar]
- White B. A., Paone D. A., Cacciapuoti A. F., Fricke R. J., Mosbach E. H., Hylemon P. B. Regulation of bile acid 7-dehydroxylase activity by NAD+ and NADH in cell extracts of Eubacterium species V.P.I. 12708. J Lipid Res. 1983 Jan;24(1):20–27. [PubMed] [Google Scholar]


