Abstract
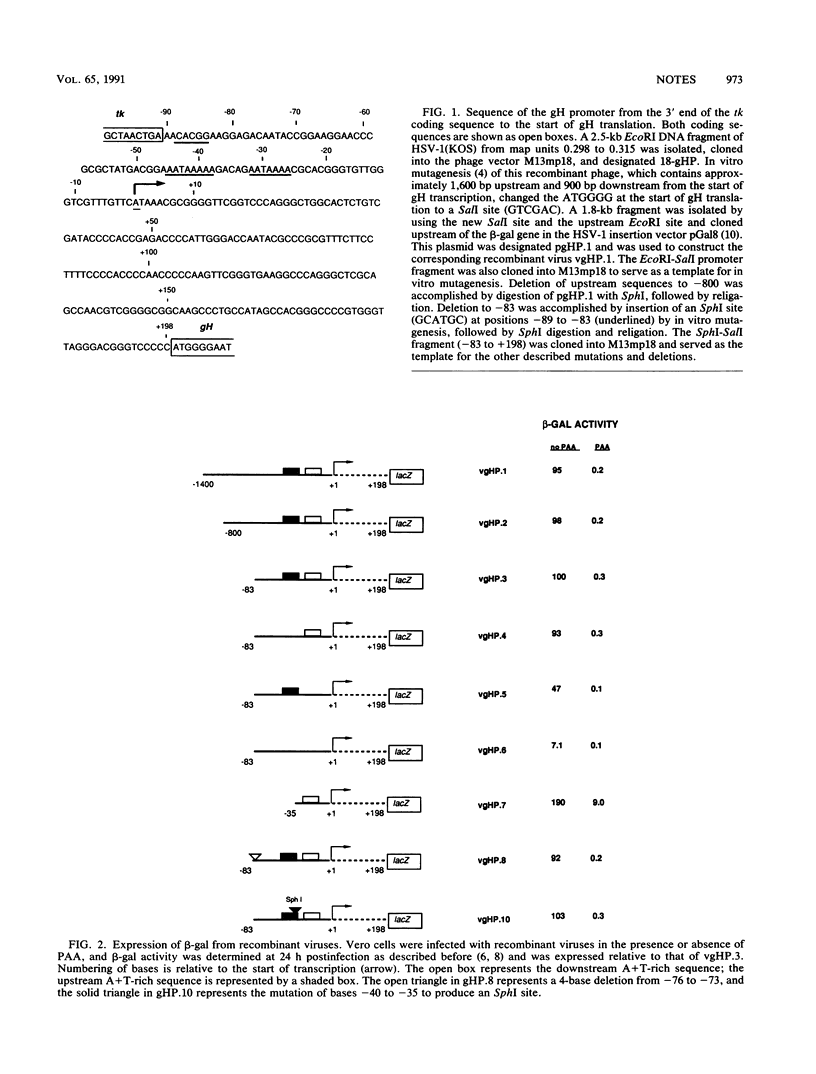
To investigate the cis-acting sequence elements that are involved in the regulation of herpes simplex virus type 1 late-gene expression, recombinant viruses were constructed that express the Escherichia coli lacZ gene from the promoter of the glycoprotein H (gH) gene. Deletion experiments established an upstream boundary for the gH promoter of no more than 83 bp from the start of gH transcription and showed that the promoter sequences did not overlap with coding sequences of the upstream thymidine kinase (tk) gene. Sequences of the tk gene previously shown to be required for efficient processing of the tk transcript were essential for expression form the gH promoter and included a TATA-like element. In addition, the gH TATA element was specifically mutagenized to substitute the TATA elements of immediate-early, early, and other late viral promoters for the gH TATA element. The results indicated that the TATA element was an interchangeable component of herpes simplex virus type 1 promoters and did not regulate temporal expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole C. N., Stacy T. P. Identification of sequences in the herpes simplex virus thymidine kinase gene required for efficient processing and polyadenylation. Mol Cell Biol. 1985 Aug;5(8):2104–2113. doi: 10.1128/mcb.5.8.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa F. L., Glorioso J. C., Levine M. A specific 15-bp TATA box promoter element is required for expression of a herpes simplex virus type 1 late gene. Genes Dev. 1988 Jan;2(1):40–53. doi: 10.1101/gad.2.1.40. [DOI] [PubMed] [Google Scholar]
- Homa F. L., Otal T. M., Glorioso J. C., Levine M. Transcriptional control signals of a herpes simplex virus type 1 late (gamma 2) gene lie within bases -34 to +124 relative to the 5' terminus of the mRNA. Mol Cell Biol. 1986 Nov;6(11):3652–3666. doi: 10.1128/mcb.6.11.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Mavromara-Nazos P., Roizman B. Delineation of regulatory domains of early (beta) and late (gamma 2) genes by construction of chimeric genes expressed in herpes simplex virus 1 genomes. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4071–4075. doi: 10.1073/pnas.86.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. A., Wagner M. J., Summers W. C. Transcription of herpes simplex virus genes in vivo: overlap of a late promoter with the 3' end of the early thymidine kinase gene. J Virol. 1983 Jan;45(1):10–17. doi: 10.1128/jvi.45.1.10-17.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Narayanan P. R. Expression of the herpes simplex virus type 1 glycoprotein C gene requires sequences in the 5' noncoding region of the gene. J Virol. 1990 Jan;64(1):445–449. doi: 10.1128/jvi.64.1.445-449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Narayanan P. R. The use of beta-galactosidase as a marker gene to define the regulatory sequences of the herpes simplex virus type 1 glycoprotein C gene in recombinant herpesviruses. Nucleic Acids Res. 1988 Nov 11;16(21):10267–10282. doi: 10.1093/nar/16.21.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Steffy K. R., Sethna M. An insertion vector for the analysis of gene expression during herpes simplex virus infection. Gene. 1990 May 14;89(2):271–274. doi: 10.1016/0378-1119(90)90016-k. [DOI] [PubMed] [Google Scholar]
- Zhang F., Denome R. M., Cole C. N. Fine-structure analysis of the processing and polyadenylation region of the herpes simplex virus type 1 thymidine kinase gene by using linker scanning, internal deletion, and insertion mutations. Mol Cell Biol. 1986 Dec;6(12):4611–4623. doi: 10.1128/mcb.6.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]



