Abstract
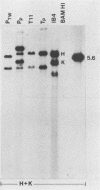
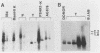
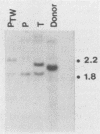
Two organ transplant recipients who received organs from a common donor and were diagnosed as having an Epstein-Barr virus (EBV)-associated posttransplant lymphoproliferative disorder were studied to determine the mode of EBV transmission. The results of restriction fragment length polymorphism, polymerase chain reaction, and minisatellite DNA analyses demonstrate that both patients had a common strain of EBV and that this strain was transmitted from the donor's organs to both recipients. Posttransplant lymphoproliferative disorder resulted from the proliferation of EBV-immortalized B lymphocytes of the recipient, not those of the donor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Chen J. D., Neilson K., Van Dyke T. Lymphotropic papovavirus early region is specifically regulated transgenic mice and efficiently induces neoplasia. J Virol. 1989 May;63(5):2204–2214. doi: 10.1128/jvi.63.5.2204-2214.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer J. S., Armstrong J., Somers J., Kusne S., Carpenter B. J., Rosenthal J. T., Ho M. Transmission of infection with herpes simplex virus by renal transplantation. J Infect Dis. 1987 Feb;155(2):202–206. doi: 10.1093/infdis/155.2.202. [DOI] [PubMed] [Google Scholar]
- Dummer J. S., Erb S., Breinig M. K., Ho M., Rinaldo C. R., Jr, Gupta P., Ragni M. V., Tzakis A., Makowka L., Van Thiel D. Infection with human immunodeficiency virus in the Pittsburgh transplant population. A study of 583 donors and 1043 recipients, 1981-1986. Transplantation. 1989 Jan;47(1):134–140. doi: 10.1097/00007890-198901000-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratama J. W., Oosterveer M. A., Lepoutre J. M., van Rood J. J., Zwaan F. E., Vossen J. M., Kapsenberg J. G., Richel D., Klein G., Ernberg I. Serological and molecular studies of Epstein-Barr virus infection in allogeneic marrow graft recipients. Transplantation. 1990 Apr;49(4):725–730. doi: 10.1097/00007890-199004000-00014. [DOI] [PubMed] [Google Scholar]
- Gratama J. W., Oosterveer M. A., Zwaan F. E., Lepoutre J., Klein G., Ernberg I. Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8693–8696. doi: 10.1073/pnas.85.22.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanto D. W., Frizzera G., Gajl-Peczalska K. J., Simmons R. L. Epstein-Barr virus, immunodeficiency, and B cell lymphoproliferation. Transplantation. 1985 May;39(5):461–472. doi: 10.1097/00007890-198505000-00001. [DOI] [PubMed] [Google Scholar]
- Ho M., Jaffe R., Miller G., Breinig M. K., Dummer J. S., Makowka L., Atchison R. W., Karrer F., Nalesnik M. A., Starzl T. E. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestations in children. Transplantation. 1988 Apr;45(4):719–727. doi: 10.1097/00007890-198804000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Suwansirikul S., Dowling J. N., Youngblood L. A., Armstrong J. A. The transplanted kidney as a source of cytomegalovirus infection. N Engl J Med. 1975 Nov 27;293(22):1109–1112. doi: 10.1056/NEJM197511272932201. [DOI] [PubMed] [Google Scholar]
- Horwitz C. A., Henle W., Henle G., Rudnick H., Latts E. Long-term serological follow-up of patients for Epstein-Barr virus after recovery from infectious mononucleosis. J Infect Dis. 1985 Jun;151(6):1150–1153. doi: 10.1093/infdis/151.6.1150. [DOI] [PubMed] [Google Scholar]
- Lung M. L., Chang R. S., Jones J. H. Genetic polymorphism of natural Epstein-Barr virus isolates from infectious mononucleosis patients and healthy carriers. J Virol. 1988 Oct;62(10):3862–3866. doi: 10.1128/jvi.62.10.3862-3866.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalesnik M. A., Jaffe R., Starzl T. E., Demetris A. J., Porter K., Burnham J. A., Makowka L., Ho M., Locker J. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988 Oct;133(1):173–192. [PMC free article] [PubMed] [Google Scholar]
- Raab-Traub N., Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986 Dec 26;47(6):883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- Rocchi G., Felici A., Ragona G., Heinz A. Quantitative evaluation of Epstein-Barr-virus-infected mononuclear peripheral blood leukocytes in infectious mononucleosis. N Engl J Med. 1977 Jan 20;296(3):132–134. doi: 10.1056/NEJM197701202960302. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Nalesnik M. A., Porter K. A., Ho M., Iwatsuki S., Griffith B. P., Rosenthal J. T., Hakala T. R., Shaw B. W., Jr, Hardesty R. L. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984 Mar 17;1(8377):583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisetschlaeger M., Yandava C. N., Furmanski L. A., Strominger J. L., Speck S. H. Promoter switching in Epstein-Barr virus during the initial stages of infection of B lymphocytes. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1725–1729. doi: 10.1073/pnas.87.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q. Y., Ogan P., Rowe M., Wood M., Rickinson A. B. Epstein-Barr virus-infected B cells persist in the circulation of acyclovir-treated virus carriers. Int J Cancer. 1989 Jan 15;43(1):67–71. doi: 10.1002/ijc.2910430115. [DOI] [PubMed] [Google Scholar]
- Zutter M. M., Martin P. J., Sale G. E., Shulman H. M., Fisher L., Thomas E. D., Durnam D. M. Epstein-Barr virus lymphoproliferation after bone marrow transplantation. Blood. 1988 Aug;72(2):520–529. [PubMed] [Google Scholar]
- de-Thé G., Geser A., Day N. E., Tukei P. M., Williams E. H., Beri D. P., Smith P. G., Dean A. G., Bronkamm G. W., Feorino P. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature. 1978 Aug 24;274(5673):756–761. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]