Abstract
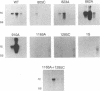
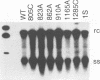
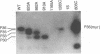
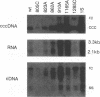
The envelope gene of the avian hepadnavirus, duck hepatitis B virus, was mutated in order to dissect the functions of the two major envelope proteins pre-S/S and S. Both envelope proteins were found to be required for virus particle assembly and secretion. The placement of stop codons after each of the first three AUG codons in the pre-S region allowed efficient translational initiation at downstream AUG codons to produce novel N-terminally truncated pre-S/S proteins. These proteins could substitute for pre-S/S protein in the production of enveloped virus production, but not in the production infectious virus. A mutant defective in myristylation of the pre-S/S protein produced reduced amounts of enveloped virus, and this virus was not infectious. Mutants defective in the pre-S/S protein accumulated high levels of covalently closed circular viral DNA (cccDNA) compared with the wild type or with a mutant defective in only the S protein. Hyperamplification of cccDNA resulted in high levels of viral RNA, consistent with the proposed role of cccDNA as the transcriptional template. Myristylation of the pre-S/S protein was not required for control of cccDNA amplification, and mutants that produced N-terminally truncated pre-S/S proteins displayed higher levels of cccDNA. We concluded that the pre-S/S protein, but not the S protein, is required for control of cccDNA amplification and persistent infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley R. P. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988 May 15;61(10):1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Büscher M., Reiser W., Will H., Schaller H. Transcripts and the putative RNA pregenome of duck hepatitis B virus: implications for reverse transcription. Cell. 1985 Mar;40(3):717–724. doi: 10.1016/0092-8674(85)90220-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Condreay L. D., Aldrich C. E., Coates L., Mason W. S., Wu T. T. Efficient duck hepatitis B virus production by an avian liver tumor cell line. J Virol. 1990 Jul;64(7):3249–3258. doi: 10.1128/jvi.64.7.3249-3258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M. F., Pourcel C., Rousset S., Chany C., Tiollais P. Excretion of hepatitis B surface antigen particles from mouse cells transformed with cloned viral DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4549–4553. doi: 10.1073/pnas.77.8.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Furtak K., Pugh J., Summers J. Synthesis of hepadnavirus particles that contain replication-defective duck hepatitis B virus genomes in cultured HuH7 cells. J Virol. 1990 Feb;64(2):642–650. doi: 10.1128/jvi.64.2.642-650.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi T., Nomura K., Hirayama Y., Kitagawa T. Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH. Cancer Res. 1987 Aug 15;47(16):4460–4464. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuroki K., Russnak R., Ganem D. Novel N-terminal amino acid sequence required for retention of a hepatitis B virus glycoprotein in the endoplasmic reticulum. Mol Cell Biol. 1989 Oct;9(10):4459–4466. doi: 10.1128/mcb.9.10.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion P. L., Knight S. S., Feitelson M. A., Oshiro L. S., Robinson W. S. Major polypeptide of duck hepatitis B surface antigen particles. J Virol. 1983 Nov;48(2):534–541. doi: 10.1128/jvi.48.2.534-541.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion P. L., Oshiro L. S., Regnery D. C., Scullard G. H., Robinson W. S. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2941–2945. doi: 10.1073/pnas.77.5.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Aldrich C., Summers J., Taylor J. M. Asymmetric replication of duck hepatitis B virus DNA in liver cells: Free minus-strand DNA. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3997–4001. doi: 10.1073/pnas.79.13.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Halpern M. S., England J. M., Seal G., Egan J., Coates L., Aldrich C., Summers J. Experimental transmission of duck hepatitis B virus. Virology. 1983 Dec;131(2):375–384. doi: 10.1016/0042-6822(83)90505-6. [DOI] [PubMed] [Google Scholar]
- Mason W. S., Seal G., Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980 Dec;36(3):829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Bao H., Shih C., Tahara S. M. Preferred translation of human hepatitis B virus polymerase from core protein- but not from precore protein-specific transcript. J Virol. 1990 Sep;64(9):4578–4581. doi: 10.1128/jvi.64.9.4578-4581.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S., Berg P. Termination-reinitiation occurs in the translation of mammalian cell mRNAs. Mol Cell Biol. 1986 Jul;6(7):2695–2703. doi: 10.1128/mcb.6.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. A frameshift mutation in the pre-S region of the human hepatitis B virus genome allows production of surface antigen particles but eliminates binding to polymerized albumin. Proc Natl Acad Sci U S A. 1985 May;82(10):3440–3444. doi: 10.1073/pnas.82.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. The preS1 protein of hepatitis B virus is acylated at its amino terminus with myristic acid. J Virol. 1987 May;61(5):1672–1677. doi: 10.1128/jvi.61.5.1672-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petcu D. J., Aldrich C. E., Coates L., Taylor J. M., Mason W. S. Suramin inhibits in vitro infection by duck hepatitis B virus, Rous sarcoma virus, and hepatitis delta virus. Virology. 1988 Dec;167(2):385–392. [PubMed] [Google Scholar]
- Pugh J. C., Sninsky J. J., Summers J. W., Schaeffer E. Characterization of a pre-S polypeptide on the surfaces of infectious avian hepadnavirus particles. J Virol. 1987 May;61(5):1384–1390. doi: 10.1128/jvi.61.5.1384-1390.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh J. C., Summers J. W. Infection and uptake of duck hepatitis B virus by duck hepatocytes maintained in the presence of dimethyl sulfoxide. Virology. 1989 Oct;172(2):564–572. doi: 10.1016/0042-6822(89)90199-2. [DOI] [PubMed] [Google Scholar]
- Schlicht H. J., Kuhn C., Guhr B., Mattaliano R. J., Schaller H. Biochemical and immunological characterization of the duck hepatitis B virus envelope proteins. J Virol. 1987 Jul;61(7):2280–2285. doi: 10.1128/jvi.61.7.2280-2285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R., Kaleta E. F., Will H. Isolation and characterization of a hepatitis B virus endemic in herons. J Virol. 1988 Oct;62(10):3832–3839. doi: 10.1128/jvi.62.10.3832-3839.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Summers J., Smith P. M., Horwich A. L. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990 Jun;64(6):2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Tuttleman J. S., Pourcel C., Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986 Nov 7;47(3):451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- Tuttleman J. S., Pugh J. C., Summers J. W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986 Apr;58(1):17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Coates L., Aldrich C. E., Summers J., Mason W. S. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990 Mar;175(1):255–261. doi: 10.1016/0042-6822(90)90206-7. [DOI] [PubMed] [Google Scholar]
- Yokosuka O., Omata M., Ito Y. Expression of pre-S1, pre-S2, and C proteins in duck hepatitis B virus infection. Virology. 1988 Nov;167(1):82–86. doi: 10.1016/0042-6822(88)90056-6. [DOI] [PubMed] [Google Scholar]