Abstract
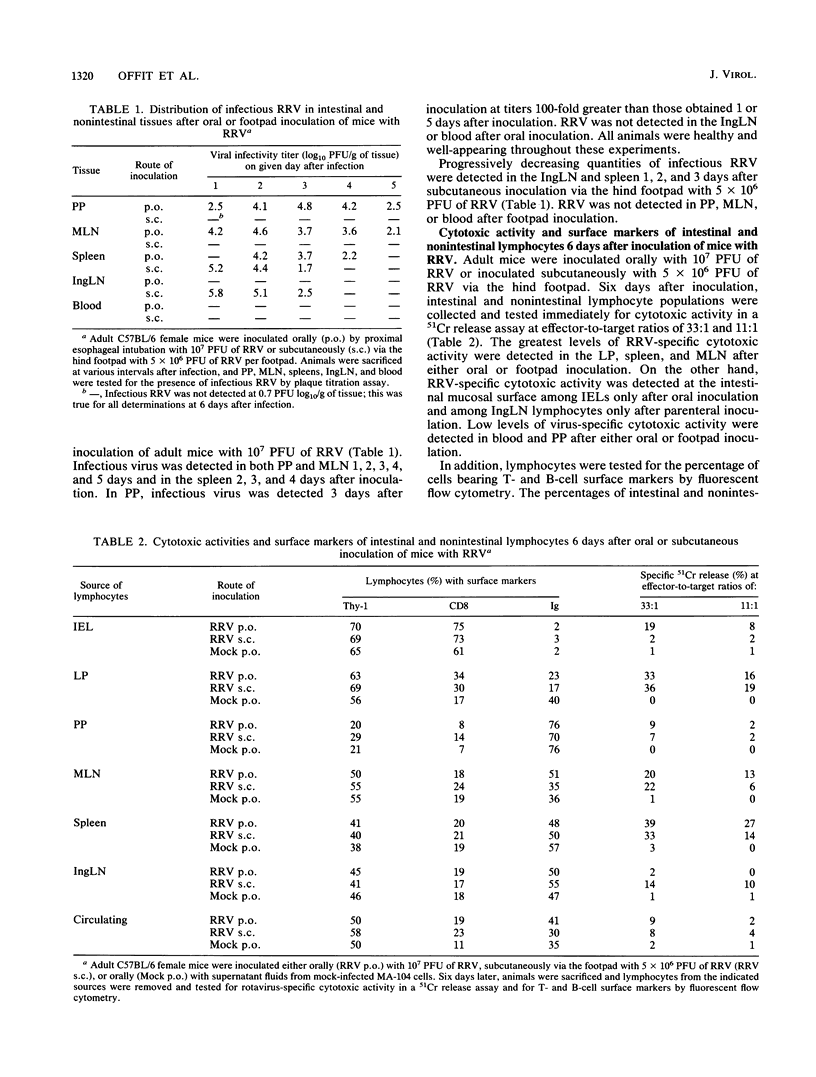
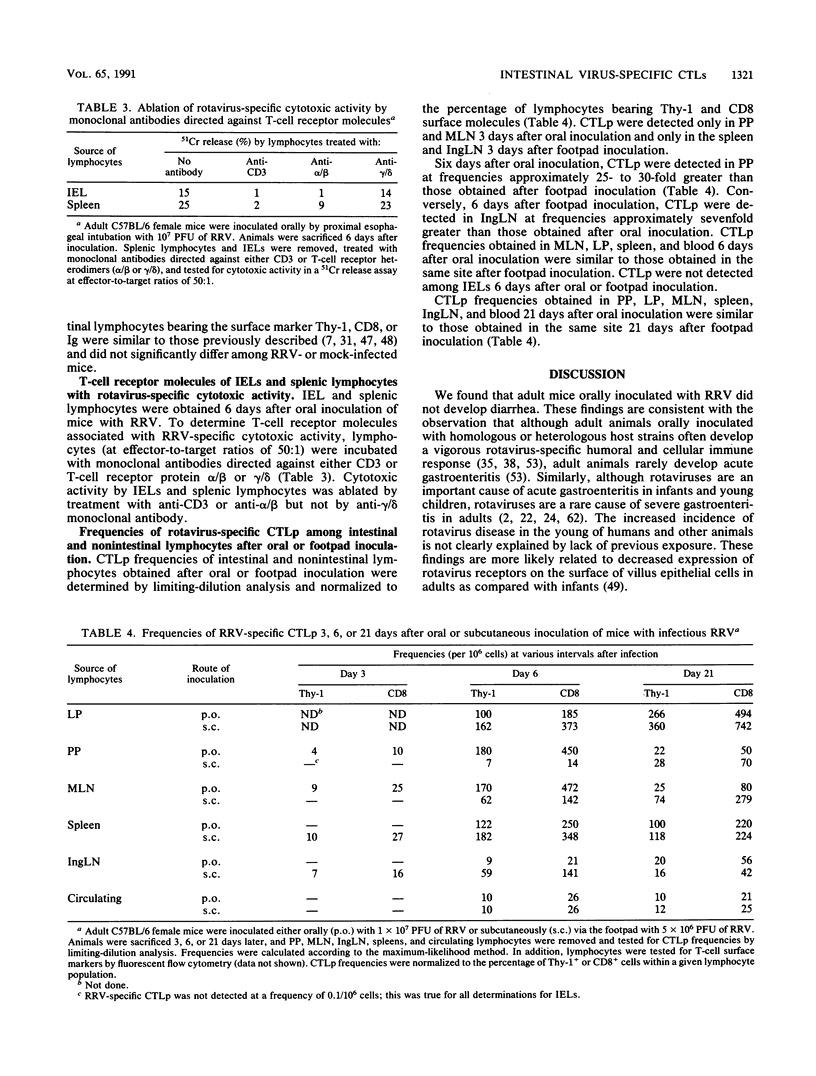
The gastrointestinal tract is constantly exposed to a variety of potentially invasive bacteria, viruses, and parasites. The first line of defense against these pathogens is the intestinal mucosal surface, which consists of epithelial cells, intraepithelial lymphocytes (IELs), mucus, and secretory immunoglobulins. In addition, the intestine is a rich source of lymphocytes located within Peyer's patches and the lamina propria. Little is known about the function, memory, trafficking, or origin of intestinal T lymphocytes after intestinal infection. We studied the murine cytotoxic T-lymphocyte (CTL) response to the intestinal pathogen rotavirus (simian strain RRV). Adult mice were inoculated orally or via the hind footpad with RRV; virus-specific cytotoxic activities in intestinal and nonintestinal lymphocyte populations were determined by 51Cr release assays. In addition, virus-specific CTL precursor (CTLp) frequencies were determined by limiting-dilution analysis. IELs containing rotavirus-specific cytotoxic activity were detected after oral but not footpad inoculation and expressed alpha/beta but not gamma/delta cell surface protein; virus-specific CTLs did not appear to arise from CTLp among IELs. In addition, the site at which RRV was presented to the immune system determined the site at which RRV-specific CTLp first appeared. Frequencies of rotavirus-specific CTLp detected in Peyer's patches were 25- to 30-fold greater after oral than after footpad inoculation. However, regardless of the route of inoculation, rotavirus-specific CTLp were distributed throughout the lymphoid system 21 days after infection. Implications of these findings for vaccine design are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baine Y., Thorbecke G. J. Induction and persistence of local B cell memory in mice. J Immunol. 1982 Feb;128(2):639–643. [PubMed] [Google Scholar]
- Barrón-Romero B. L., Barreda-González J., Doval-Ugalde R., Zermeño-Eguia Liz J., Huerta-Peña M. Asymptomatic rotavirus infections in day care centers. J Clin Microbiol. 1985 Jul;22(1):116–118. doi: 10.1128/jcm.22.1.116-118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E. L., Goldstein L. A., Jutila M. A., Nakache M., Picker L. J., Streeter P. R., Wu N. W., Zhou D., Butcher E. C. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol Rev. 1989 Apr;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Black R. E., Merson M. H., Rahman A. S., Yunus M., Alim A. R., Huq I., Yolken R. H., Curlin G. T. A two-year study of bacterial, viral, and parasitic agents associated with diarrhea in rural Bangladesh. J Infect Dis. 1980 Nov;142(5):660–664. doi: 10.1093/infdis/142.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M., Janeway C. A., Jr, Ito K., Haser W., Ishida I., Nakanishi N., Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature. 1988 Dec 1;336(6198):479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus. VI. Migration and activity in vivo in acute and persistent infection. J Immunol. 1986 Jan;136(2):698–704. [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D., Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985 Jan;26(1):81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner M. E., Estes M. K., Graham D. Y. Rabbit model of rotavirus infection. J Virol. 1988 May;62(5):1625–1633. doi: 10.1128/jvi.62.5.1625-1633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharakul T., Riepenhoff-Talty M., Albini B., Ogra P. L. Distribution of rotavirus antigen in intestinal lymphoid tissues: potential role in development of the mucosal immune response to rotavirus. Clin Exp Immunol. 1988 Oct;74(1):14–19. [PMC free article] [PubMed] [Google Scholar]
- Dharakul T., Rott L., Greenberg H. B. Recovery from chronic rotavirus infection in mice with severe combined immunodeficiency: virus clearance mediated by adoptive transfer of immune CD8+ T lymphocytes. J Virol. 1990 Sep;64(9):4375–4382. doi: 10.1128/jvi.64.9.4375-4382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. Specific immune lysis of paramyxovirus-infected cells by H-2-compatible thymus-derived lymphocytes. Immunology. 1976 Jul;31(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- Dunkley M. L., Husband A. J. The induction and migration of antigen-specific helper cells for IgA responses in the intestine. Immunology. 1986 Mar;57(3):379–385. [PMC free article] [PubMed] [Google Scholar]
- Edmondson W. P., Purcell R. H., Gundelfinger B. F., Love J. W., Ludwig W., Chanock R. M. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue culture. II. specific protective effect against epidemic disease. JAMA. 1966 Feb 7;195(6):453–459. [PubMed] [Google Scholar]
- Ennis F. A., Wells M. A., Butchko G. M., Albrecht P. Evidence that cytotoxic T cells are part of the host's response to influenza pneumonia. J Exp Med. 1978 Nov 1;148(5):1241–1250. doi: 10.1084/jem.148.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas de St Groth The evaluation of limiting dilution assays. J Immunol Methods. 1982 Mar 12;49(2):R11–R23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S., Nakanishi N., Kanagawa O., Kubo R., Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor gamma delta: analysis of gamma delta T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutila M. A., Berg E. L., Kishimoto T. K., Picker L. J., Bargatze R. F., Bishop D. K., Orosz C. G., Wu N. W., Butcher E. C. Inflammation-induced endothelial cell adhesion to lymphocytes, neutrophils, and monocytes. Role of homing receptors and other adhesion molecules. Transplantation. 1989 Nov;48(5):727–731. doi: 10.1097/00007890-198911000-00001. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Hoshino Y., Glass R. I., Midthun K., Gorziglia M., Chanock R. M. Rotavirus: the major etiologic agent of severe infantile diarrhea may be controllable by a "Jennerian" approach to vaccination. J Infect Dis. 1986 May;153(5):815–822. doi: 10.1093/infdis/153.5.815. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Levine M. M., Yolken R. H., VanKirk D. H., Dolin R., Greenberg H. B., Chanock R. M. Oral administration of human rotavirus to volunteers: induction of illness and correlates of resistance. J Infect Dis. 1983 Jan;147(1):95–106. doi: 10.1093/infdis/147.1.95. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Brandt C. D., Kapikian A. Z., Wyatt R. G., Arrobio J. O., Rodriguez W. J., Chanock R. M., Parrott R. H. Human reovirus-like agent infection. Occurrence in adult contacts of pediatric patients with gastroenteritis. JAMA. 1977 Aug 1;238(5):404–407. doi: 10.1001/jama.238.5.404. [DOI] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb M. F., Lyons C. R., O'Hara R. M., Jr, Stein-Streilein J. The antigen-induced selective recruitment of specific T lymphocytes to the lung. J Immunol. 1982 Jan;128(1):111–115. [PubMed] [Google Scholar]
- London S. D., Cebra J. J., Rubin D. H. Intraepithelial lymphocytes contain virus-specific, MHC-restricted cytotoxic cell precursors after gut mucosal immunization with reovirus serotype 1/Lang. Reg Immunol. 1989 Mar-Apr;2(2):98–102. [PubMed] [Google Scholar]
- London S. D., Rubin D. H., Cebra J. J. Gut mucosal immunization with reovirus serotype 1/L stimulates virus-specific cytotoxic T cell precursors as well as IgA memory cells in Peyer's patches. J Exp Med. 1987 Mar 1;165(3):830–847. doi: 10.1084/jem.165.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Lim Y., Krall G., Kapikian A. Z., Levine M. M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988 Jun;7(6):388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- McNabb P. C., Tomasi T. B. Host defense mechanisms at mucosal surfaces. Annu Rev Microbiol. 1981;35:477–496. doi: 10.1146/annurev.mi.35.100181.002401. [DOI] [PubMed] [Google Scholar]
- McWilliams M., Lamm M. E., Phillips-Quagliata J. M. Surface and intracellular markers of mouse mesenteric and peripheral lymph node and Peyer's patch cells. J Immunol. 1974 Oct;113(4):1326–1333. [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Kornstein M. J., Plotkin S. A. A murine model for oral infection with a primate rotavirus (simian SA11). J Virol. 1984 Jul;51(1):233–236. doi: 10.1128/jvi.51.1.233-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Plotkin S. A. Response of mice to rotaviruses of bovine or primate origin assessed by radioimmunoassay, radioimmunoprecipitation, and plaque reduction neutralization. Infect Immun. 1983 Oct;42(1):293–300. doi: 10.1128/iai.42.1.293-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes appear at the intestinal mucosal surface after rotavirus infection. J Virol. 1989 Aug;63(8):3507–3512. doi: 10.1128/jvi.63.8.3507-3512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes cross-react with target cells infected with different rotavirus serotypes. J Virol. 1988 Jan;62(1):127–131. doi: 10.1128/jvi.62.1.127-131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes passively protect against gastroenteritis in suckling mice. J Virol. 1990 Dec;64(12):6325–6328. doi: 10.1128/jvi.64.12.6325-6328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Svoboda Y. M. Rotavirus-specific cytotoxic T lymphocyte response of mice after oral inoculation with candidate rotavirus vaccine strains RRV or WC3. J Infect Dis. 1989 Nov;160(5):783–788. doi: 10.1093/infdis/160.5.783. [DOI] [PubMed] [Google Scholar]
- Ottaway C. A., Manson-Smith D. F., Bruce R. G., Parrott D. M. Regional blood flow and the localization of lymphoblasts in the small intestine of the mouse. II. The effects of a primary enteric infection with Trichinella spiralis. Immunology. 1980 Dec;41(4):963–971. [PMC free article] [PubMed] [Google Scholar]
- Ottaway C. A., Parrott D. M. Regional blood flow and the localization of lymphoblasts in the small intestine of the mouse. I. Examination of normal small intestine. Immunology. 1980 Dec;41(4):955–961. [PMC free article] [PubMed] [Google Scholar]
- Pabst R., Reynolds J. D. Evidence of extensive lymphocyte death in sheep Peyer's patches. II. The number and fate of newly-formed lymphocytes that emigrate from Peyer's patches. J Immunol. 1986 Mar 15;136(6):2011–2017. [PubMed] [Google Scholar]
- Pabst R. The spleen in lymphocyte migration. Immunol Today. 1988 Feb;9(2):43–45. doi: 10.1016/0167-5699(88)91258-3. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., Tait C., MacKenzie S., Mowat A. M., Davies M. D., Micklem H. S. Analysis of the effector functions of different populations of mucosal lymphocytes. Ann N Y Acad Sci. 1983 Jun 30;409:307–320. doi: 10.1111/j.1749-6632.1983.tb26879.x. [DOI] [PubMed] [Google Scholar]
- Petit A., Ernst P. B., Befus A. D., Clark D. A., Rosenthal K. L., Ishizaka T., Bienenstock J. Murine intestinal intraepithelial lymphocytes I. Relationship of a novel Thy-1-,Lyt-1-,Lyt-2+, granulated subpopulation to natural killer cells and mast cells. Eur J Immunol. 1985 Mar;15(3):211–215. doi: 10.1002/eji.1830150302. [DOI] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Dharakul T., Kowalski E., Michalak S., Ogra P. L. Persistent rotavirus infection in mice with severe combined immunodeficiency. J Virol. 1987 Oct;61(10):3345–3348. doi: 10.1128/jvi.61.10.3345-3348.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Lee P. C., Carmody P. J., Barrett H. J., Ogra P. L. Age-dependent rotavirus-enterocyte interactions. Proc Soc Exp Biol Med. 1982 Jun;170(2):146–154. doi: 10.3181/00379727-170-41410. [DOI] [PubMed] [Google Scholar]
- Rupprecht C. E., Hamir A. N., Johnston D. H., Koprowski H. Efficacy of a vaccinia-rabies glycoprotein recombinant virus vaccine in raccoons (Procyon lotor). Rev Infect Dis. 1988 Nov-Dec;10 (Suppl 4):S803–S809. doi: 10.1093/clinids/10.supplement_4.s803. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Sheridan J. F., Eydelloth R. S., Vonderfecht S. L., Aurelian L. Virus-specific immunity in neonatal and adult mouse rotavirus infection. Infect Immun. 1983 Feb;39(2):917–927. doi: 10.1128/iai.39.2.917-927.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey W. G., Collins J., Wallis T. S., Clarke G. J., Spencer A. J., Haddon S. J., Osborne M. P., Candy D. C., Stephen J. Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J Gen Virol. 1986 Dec;67(Pt 12):2625–2634. doi: 10.1099/0022-1317-67-12-2625. [DOI] [PubMed] [Google Scholar]
- Streeter P. R., Rouse B. T., Butcher E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988 Nov;107(5):1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober S., Dilley J. Maturation of B lymphocytes in the rat. I. Migration pattern, tissue distribution, and turnover rate of unprimed and primed B lymphocytes involved in the adoptive antidinitrophenyl response. J Exp Med. 1973 Dec 1;138(6):1331–1344. doi: 10.1084/jem.138.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totterdell B. M., Banatvala J. E., Chrystie I. L., Ball G., Cubitt W. D. Systemic lymphoproliferative responses to rotavirus. J Med Virol. 1988 May;25(1):37–44. doi: 10.1002/jmv.1890250106. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Riepenhoff-Talty M., Dharakul T., Chegas P., Fisher J. E., Greenberg H. B., Ogra P. L. Extramucosal spread and development of hepatitis in immunodeficient and normal mice infected with rhesus rotavirus. J Virol. 1990 Jan;64(1):361–368. doi: 10.1128/jvi.64.1.361-368.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Stone J., Lazzell V., Bergmann K. C., Khakoo R., Jacknowitz A., Howard S., Rose C. Oral route as method for immunizing against mucosal pathogens. Ann N Y Acad Sci. 1983 Jun 30;409:510–516. doi: 10.1111/j.1749-6632.1983.tb26895.x. [DOI] [PubMed] [Google Scholar]
- Walsh J. A., Warren K. S. Selective primary health care: an interim strategy for disease control in developing countries. N Engl J Med. 1979 Nov 1;301(18):967–974. doi: 10.1056/NEJM197911013011804. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Ennis F. A., Albrecht P. Recovery from a viral respiratory infection. II. Passive transfer of immune spleen cells to mice with influenza pneumonia. J Immunol. 1981 Mar;126(3):1042–1046. [PubMed] [Google Scholar]
- Wenman W. M., Hinde D., Feltham S., Gurwith M. Rotavirus infection in adults. Results of a prospective family study. N Engl J Med. 1979 Aug 9;301(6):303–306. doi: 10.1056/NEJM197908093010604. [DOI] [PubMed] [Google Scholar]
- Wolf J. L., Dambrauskas R., Sharpe A. H., Trier J. S. Adherence to and penetration of the intestinal epithelium by reovirus type 1 in neonatal mice. Gastroenterology. 1987 Jan;92(1):82–91. doi: 10.1016/0016-5085(87)90842-0. [DOI] [PubMed] [Google Scholar]


