Abstract
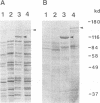
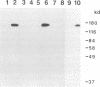
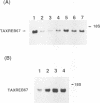
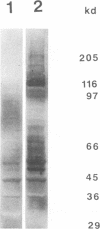
One of the gene products of human T-cell leukemia virus type I (HTLV-I), p40tax, activates its own viral transcription in trans through tax-responsive enhancers in viral long terminal repeats. Five species of cDNA clones for proteins that bind to the tax-responsive enhancer element in HTLV-I were isolated from the Jurkat cell library. The beta-galactosidase fusion protein prepared from the lysogen of a clone specifically recognized the cyclic AMP-responsive element in HTLV-I enhancer. The nucleotide sequence of a full-length cDNA clone (TAXREB67) had a coding capacity of 351 amino acids, which contained a basic motif followed by a leucine zipper structure near the carboxy terminus. Its mRNA was detected in human cell lines, including HTLV-I-infected or noninfected hematopoietic cell lines. The mRNA level in Jurkat cells was decreased temporarily by increasing cyclic AMP concentration but increased by increasing Ca2+ concentration. Polyclonal antibodies against the fusion protein specifically recognized a 52-kDa protein in Jurkat cells. Analyses of the function of this protein and its interactions with other cellular factors will be useful to help understand the regulatory mechanism through tax-responsive enhancers in HTLV-I.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beimling P., Moelling K. Isolation and characterization of the tax protein of HTLV-I. Oncogene. 1989 Apr;4(4):511–516. [PubMed] [Google Scholar]
- Brady J., Jeang K. T., Duvall J., Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987 Jul;61(7):2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988 May;8(5):2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Dang C. V., McGuire M., Buckmire M., Lee W. M. Involvement of the 'leucine zipper' region in the oligomerization and transforming activity of human c-myc protein. Nature. 1989 Feb 16;337(6208):664–666. doi: 10.1038/337664a0. [DOI] [PubMed] [Google Scholar]
- Didier D. K., Schiffenbauer J., Woulfe S. L., Zacheis M., Schwartz B. D. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7322–7326. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M., Sassone-Corsi P., Verma I. M. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa J., Seiki M., Kiyokawa T., Yoshida M. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2277–2281. doi: 10.1073/pnas.82.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa J., Toita M., Yoshida M. A unique enhancer element for the trans activator (p40tax) of human T-cell leukemia virus type I that is distinct from cyclic AMP- and 12-O-tetradecanoylphorbol-13-acetate-responsive elements. J Virol. 1989 Aug;63(8):3234–3239. doi: 10.1128/jvi.63.8.3234-3239.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Giam C. Z., Xu Y. L. HTLV-I tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J Biol Chem. 1989 Sep 15;264(26):15236–15241. [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Halazonetis T. D., Georgopoulos K., Greenberg M. E., Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988 Dec 2;55(5):917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6442–6446. doi: 10.1073/pnas.81.20.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S. I., Ryseck R. P., Mechta F., Bravo R., Yaniv M. Characterization of junD: a new member of the jun proto-oncogene family. EMBO J. 1989 May;8(5):1433–1439. doi: 10.1002/j.1460-2075.1989.tb03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Yun Y., Jameson J. L., Habener J. F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. GCN4, a eukaryotic transcriptional activator protein, binds as a dimer to target DNA. EMBO J. 1987 Sep;6(9):2781–2784. doi: 10.1002/j.1460-2075.1987.tb02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Boros I., Brady J., Radonovich M., Khoury G. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J Virol. 1988 Dec;62(12):4499–4509. doi: 10.1128/jvi.62.12.4499-4509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadison P., Poteat H. T., Klein K. M., Faller D. V. Role of protein kinase A in tax transactivation of the human T-cell leukemia virus type I long terminal repeat. J Virol. 1990 May;64(5):2141–2148. doi: 10.1128/jvi.64.5.2141-2148.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F., Lam E., Chua N. H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature. 1989 Aug 31;340(6236):727–730. doi: 10.1038/340727a0. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lilienbaum A., Duc Dodon M., Alexandre C., Gazzolo L., Paulin D. Effect of human T-cell leukemia virus type I tax protein on activation of the human vimentin gene. J Virol. 1990 Jan;64(1):256–263. doi: 10.1128/jvi.64.1.256-263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987 Mar 13;48(5):847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Sakura H., Kanei-Ishii C., Sudo T., Yoshimura T., Fujisawa J., Yoshida M., Ishii S. Leucine zipper structure of the protein CRE-BP1 binding to the cyclic AMP response element in brain. EMBO J. 1989 Jul;8(7):2023–2028. doi: 10.1002/j.1460-2075.1989.tb03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott S. J., Boros I., Duvall J. F., Brady J. N. Indirect binding of human T-cell leukemia virus type I tax1 to a responsive element in the viral long terminal repeat. Mol Cell Biol. 1989 Oct;9(10):4152–4160. doi: 10.1128/mcb.9.10.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Yoshida M., Arai K. T-cell activation signals and human T-cell leukemia virus type I-encoded p40x protein activate the mouse granulocyte-macrophage colony-stimulating factor gene through a common DNA element. Mol Cell Biol. 1988 Dec;8(12):5581–5587. doi: 10.1128/mcb.8.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne J., Béraud C., Crenon I., Lombard-Platet G., Gazzolo L., Sergeant A., Jalinot P. Tax1 induction of the HTLV-I 21 bp enhancer requires cooperation between two cellular DNA-binding proteins. EMBO J. 1990 Mar;9(3):957–964. doi: 10.1002/j.1460-2075.1990.tb08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye-Rowley W. S., Harshman K. D., Parker C. S. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev. 1989 Mar;3(3):283–292. doi: 10.1101/gad.3.3.283. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Yoshida M., Seiki M. A single species of pX mRNA of human T-cell leukemia virus type I encodes trans-activator p40x and two other phosphoproteins. J Virol. 1986 Nov;60(2):394–399. doi: 10.1128/jvi.60.2.394-399.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Ohtani K., Nakamura M., Sugamura K. Activation of endogenous c-fos proto-oncogene expression by human T-cell leukemia virus type I-encoded p40tax protein in the human T-cell line, Jurkat. J Virol. 1989 Aug;63(8):3220–3226. doi: 10.1128/jvi.63.8.3220-3226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Nathans D. The basic region of Fos mediates specific DNA binding. EMBO J. 1989 Dec 1;8(12):3833–3841. doi: 10.1002/j.1460-2075.1989.tb08561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberg M., Schuermann M., Hunter J. B., Müller R. Two functionally different regions in Fos are required for the sequence-specific DNA interaction of the Fos/Jun protein complex. Nature. 1989 Apr 13;338(6216):589–590. doi: 10.1038/338589a0. [DOI] [PubMed] [Google Scholar]
- Nyunoya H., Ogura T., Kikuchi M., Iwamoto H., Yamashita K., Maekawa M., Takebe Y., Miyamura K., Yamazaki S., Shimotohno K. Expression of HTLV-I envelope protein fused to hydrophobic amino-terminal peptide of baculovirus polyhedrin in insect cells and its application for serological assays. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1311–1321. doi: 10.1089/aid.1990.6.1311. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986 May 3;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteat H. T., Kadison P., McGuire K., Park L., Park R. E., Sodroski J. G., Haseltine W. A. Response of the human T-cell leukemia virus type 1 long terminal repeat to cyclic AMP. J Virol. 1989 Apr;63(4):1604–1611. doi: 10.1128/jvi.63.4.1604-1611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonovich M., Jeang K. T. Activation of the human T-cell leukemia virus type I long terminal repeat by 12-O-tetradecanoylphorbol-13-acetate and by tax (p40x) occurs through similar but functionally distinct target sequences. J Virol. 1989 Jul;63(7):2987–2994. doi: 10.1128/jvi.63.7.2987-2994.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Park R., Sodroski J. G., Haseltine W. A. Multiple sequence elements are required for regulation of human T-cell leukemia virus gene expression. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4919–4923. doi: 10.1073/pnas.84.14.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lanahan A., Perez-Albuerne E., Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1500–1503. doi: 10.1073/pnas.86.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Nathans D. Induction of protooncogene c-jun by serum growth factors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8464–8467. doi: 10.1073/pnas.85.22.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura H., Maekawa T., Imamoto F., Yasuda K., Ishii S. Two human genes isolated by a novel method encode DNA-binding proteins containing a common region of homology. Gene. 1988 Dec 20;73(2):499–507. doi: 10.1016/0378-1119(88)90514-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Takano M., Teruuchi T., Miwa M. Requirement of multiple copies of a 21-nucleotide sequence in the U3 regions of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8112–8116. doi: 10.1073/pnas.83.21.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Feinberg M. B., Holbrook N., Wong-Staal F., Greene W. C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Tan T. H., Jia R., Roeder R. G. Utilization of signal transduction pathway by the human T-cell leukemia virus type I transcriptional activator tax. J Virol. 1989 Sep;63(9):3761–3768. doi: 10.1128/jvi.63.9.3761-3768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Tsujimoto A., Teruuchi T., Imamura J., Shimotohno K., Miyoshi I., Miwa M. Nucleotide sequence analysis of a provirus derived from HTLV-1-associated myelopathy (HAM). Mol Biol Med. 1988 Feb;5(1):29–42. [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Osame M., Usuku K., Matsumoto M., Igata A. Viruses detected in HTLV-I-associated myelopathy and adult T-cell leukaemia are identical on DNA blotting. Lancet. 1987 May 9;1(8541):1085–1086. doi: 10.1016/s0140-6736(87)90506-x. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Fujisawa J., Yoshida M. Multiple cDNA clones encoding nuclear proteins that bind to the tax-dependent enhancer of HTLV-1: all contain a leucine zipper structure and basic amino acid domain. EMBO J. 1990 Aug;9(8):2537–2542. doi: 10.1002/j.1460-2075.1990.tb07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M., Toschi L., Ryseck R. P., Schuermann M., Müller R., Bravo R. The product of a novel growth factor activated gene, fos B, interacts with JUN proteins enhancing their DNA binding activity. EMBO J. 1989 Mar;8(3):805–813. doi: 10.1002/j.1460-2075.1989.tb03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]