Abstract
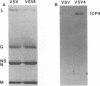
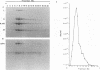
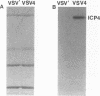
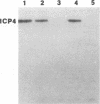
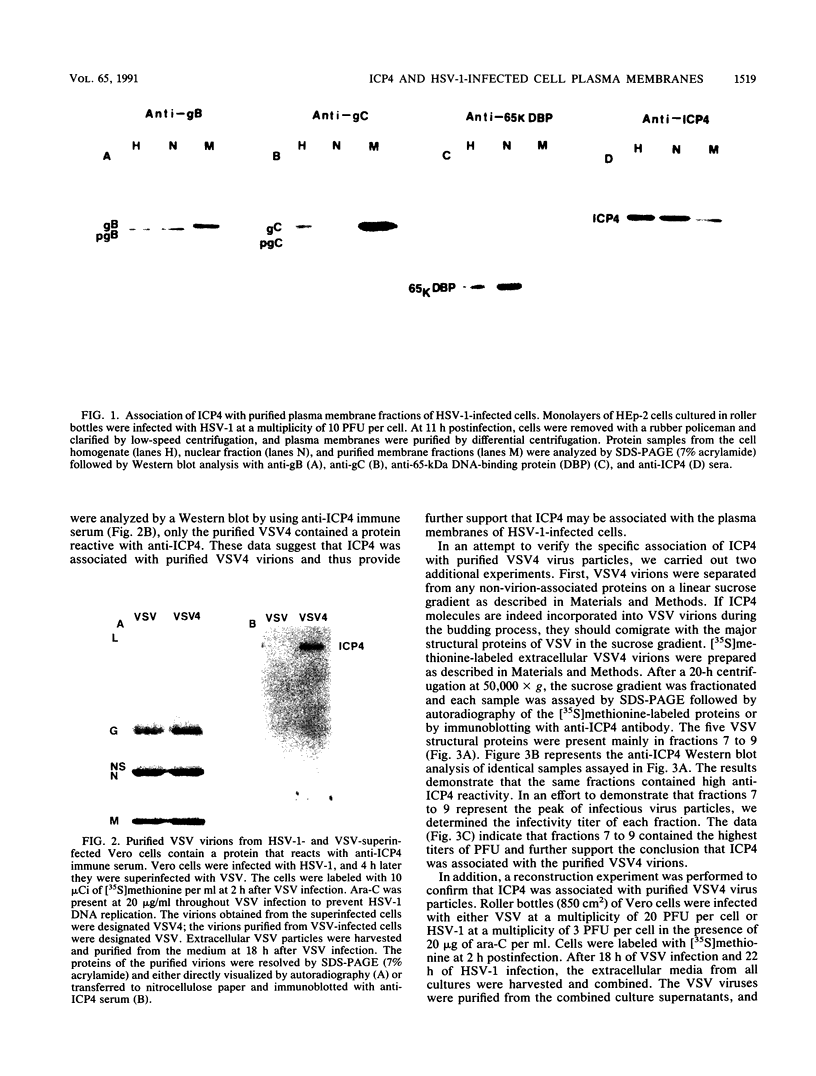
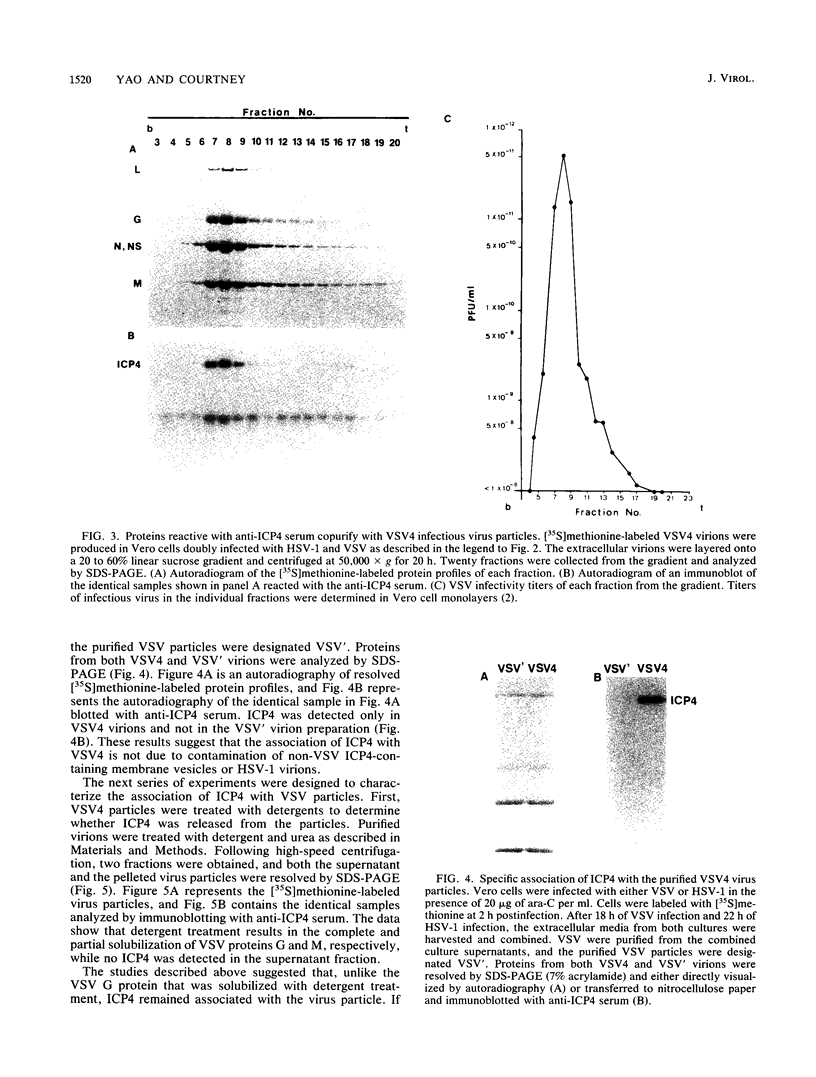
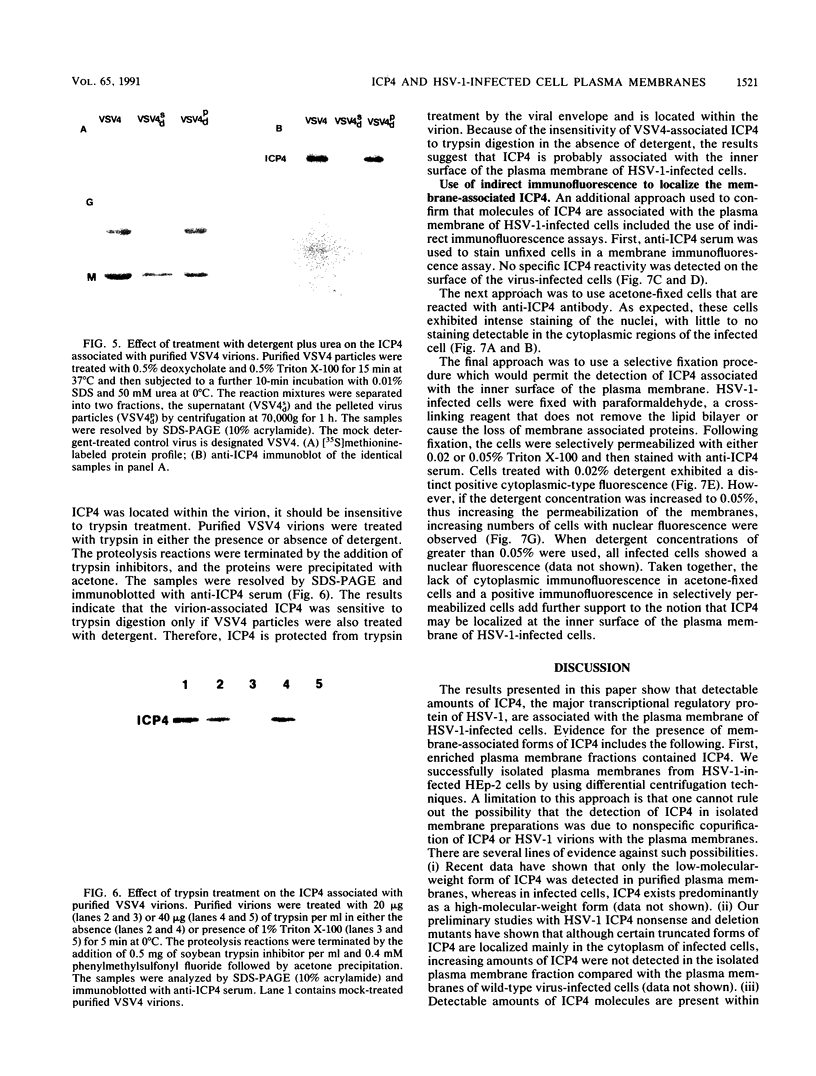
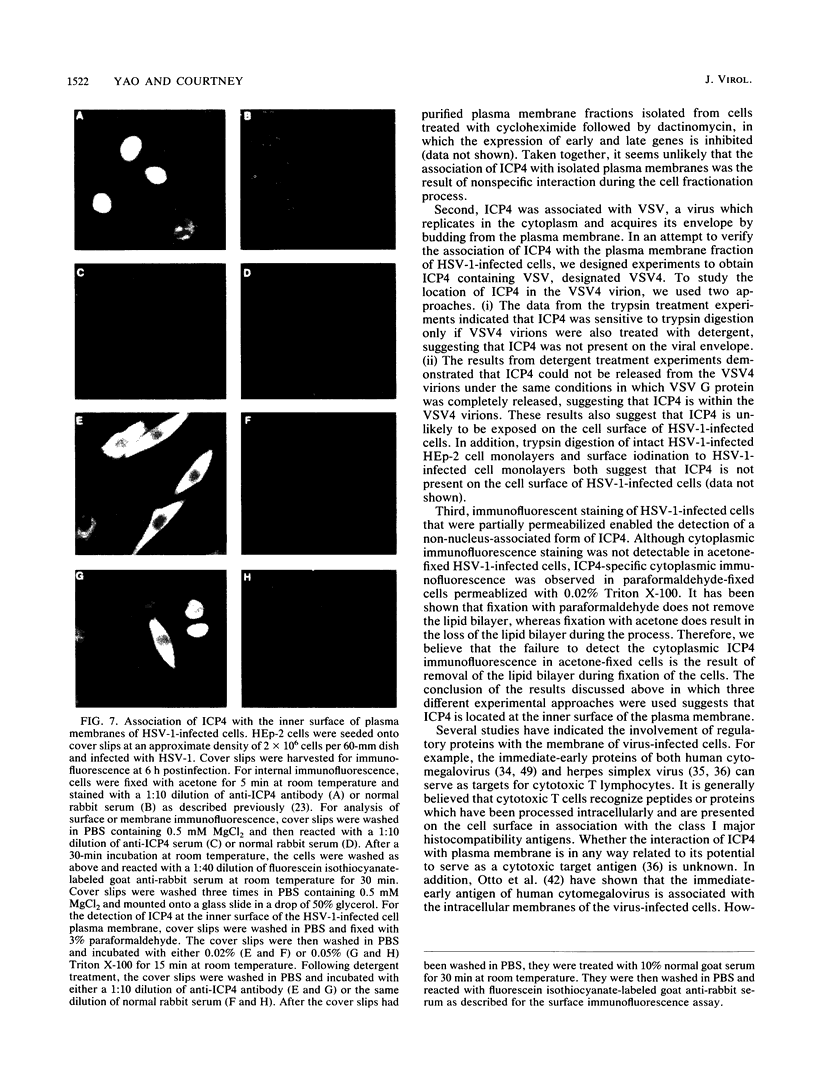
A major transcriptional regulatory protein, ICP4, of herpes simplex virus type 1 (HSV-1) is localized primarily within the nucleus soon after its synthesis. Recent studies have shown that approximately 100 to 200 molecules of ICP4 are located in the tegument region of purified virions (F. Yao and R. J. Courtney, J. Virol. 63:3338-3344, 1989). As an extension to these studies, we present data suggesting that ICP4 may also associate with the plasma membrane of HSV-1-infected cells. The experimental approaches used included the isolation and purification of plasma membranes from HSV-1-infected cells, the isolation of purified vesicular stomatitis virus containing ICP4, and immunofluorescence of HSV-1-infected cells following selective permeabilization with detergent. The results from the above studies support the suggestion that detectable amounts of ICP4 are associated with the inner surface of the plasma membrane of HSV-1-infected cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Bone D. R., Courtney R. J. A temperature-sensitive mutant of herpes simplex virus type 1 defective in the synthesis of the major capsid polypeptide. J Gen Virol. 1974 Jul;24(1):17–27. doi: 10.1099/0022-1317-24-1-17. [DOI] [PubMed] [Google Scholar]
- Cabral G. A., Courtney R. J., Schaffer P. A., Marciano-Cabral F. Ultrastructural characterization of an early, nonstructural polypeptide of herpes simplex virus type 1. J Virol. 1980 Mar;33(3):1192–1198. doi: 10.1128/jvi.33.3.1192-1198.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. Z., Schaffer P. A. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol. 1989 Nov;63(11):4579–4589. doi: 10.1128/jvi.63.11.4579-4589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. B., Watson R. J., Wilkie N. M. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell. 1977 Sep;12(1):275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- Compton T., Courtney R. J. Virus-specific glycoproteins associated with the nuclear fraction of herpes simplex virus type 1-infected cells. J Virol. 1984 Feb;49(2):594–597. doi: 10.1128/jvi.49.2.594-597.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Campadelli-Fiume G., Foa-Tomasi L., Cassai E. Evidence that herpes simplex virus DNA is transcribed by cellular RNA polymerase B. J Virol. 1977 Mar;21(3):996–1001. doi: 10.1128/jvi.21.3.996-1001.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Benyesh-Melnick M. Isolation and characterization of a large molecular-weight polypeptide of herpes simplex virus type 1. Virology. 1974 Dec;62(2):539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Courtney M. A., Schaffer P. A. Temperature-sensitive mutants in herpes simplex virus type 1 ICP4 permissive for early gene expression. J Virol. 1984 Dec;52(3):767–776. doi: 10.1128/jvi.52.3.767-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., McCarthy A. M., Schaffer P. A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985 Nov;56(2):558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol Cell Biol. 1985 Aug;5(8):1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domber E., Holowczak J. A. Vaccinia virus proteins on the plasma membranes of infected cells. IV. Studies employing L cells infected with ultraviolet-irradiated vaccinia virions. Virology. 1986 Jul 30;152(2):331–342. doi: 10.1016/0042-6822(86)90136-4. [DOI] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U. Vesicular stomatitis virus: structure and function of virion components. Curr Top Microbiol Immunol. 1976;73:1–34. doi: 10.1007/978-3-642-66306-2_1. [DOI] [PubMed] [Google Scholar]
- Everett R. D. Activation of cellular promoters during herpes virus infection of biochemically transformed cells. EMBO J. 1985 Aug;4(8):1973–1980. doi: 10.1002/j.1460-2075.1985.tb03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984 Dec 20;3(13):3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber S. W., Wilcox K. W. Association of herpes simplex virus regulatory protein ICP4 with sequences spanning the ICP4 gene transcription initiation site. Nucleic Acids Res. 1988 Jan 25;16(2):555–570. doi: 10.1093/nar/16.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber S. W., Wilcox K. W. Association of the herpes simplex virus regulatory protein ICP4 with specific nucleotide sequences in DNA. Nucleic Acids Res. 1986 Aug 11;14(15):6067–6083. doi: 10.1093/nar/14.15.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber S. W., Wilcox K. W. Characterization of a herpes simplex virus regulatory protein: aggregation and phosphorylation of a temperature-sensitive variant of ICP 4. Arch Virol. 1986;91(3-4):297–312. doi: 10.1007/BF01314289. [DOI] [PubMed] [Google Scholar]
- Fennewald S., van Santen V., Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984 Aug;51(2):411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery V. L., Courtney R. J., Schaffer P. A. Expression of an early, nonstructural antigen of herpes simplex virus in cell transformed in vitro by herpes simplex virus. J Virol. 1977 Jan;21(1):284–291. doi: 10.1128/jvi.21.1.284-291.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo M. L., Jackwood D. H., Murphy M., Marsden H. S., Parris D. S. Purification of the herpes simplex virus type 1 65-kilodalton DNA-binding protein: properties of the protein and evidence of its association with the virus-encoded DNA polymerase. J Virol. 1988 Aug;62(8):2874–2883. doi: 10.1128/jvi.62.8.2874-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman I. H., Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Lerch R. J., Earhart K. The cytoplasmic domain of herpes simplex virus type 1 glycoprotein C is required for membrane anchoring. J Virol. 1988 May;62(5):1753–1761. doi: 10.1128/jvi.62.5.1753-1761.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis D. L., Chan W. K., Estes M. K., Butel J. S. The cellular secretory pathway is not utilized for biosynthesis, modification, or intracellular transport of the simian virus 40 large tumor antigen. J Virol. 1987 Dec;61(12):3950–3959. doi: 10.1128/jvi.61.12.3950-3959.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattar-Cooley P., Wilcox K. W. Characterization of the DNA-binding properties of herpes simplex virus regulatory protein ICP4. J Virol. 1989 Feb;63(2):696–704. doi: 10.1128/jvi.63.2.696-704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Senechek D., Rice S. A., Smith J. L. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J Virol. 1987 Feb;61(2):276–284. doi: 10.1128/jvi.61.2.276-284.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U. H., Reddehase M. J., Keil G. M., Schickedanz J. Host immune response to cytomegalovirus: products of transfected viral immediate-early genes are recognized by cloned cytolytic T lymphocytes. J Virol. 1987 Jun;61(6):2054–2058. doi: 10.1128/jvi.61.6.2054-2058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Courtney R. J., Fowler G., Rouse B. T. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize virus nonstructural proteins. J Virol. 1988 Jul;62(7):2265–2273. doi: 10.1128/jvi.62.7.2265-2273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Zhu X. X., Silverstein S. J., Courtney R. J., Yao F., Jenkins F. J., Rouse B. T. Murine cytotoxic T lymphocytes specific for herpes simplex virus type 1 recognize the immediate early protein ICP4 but not ICP0. J Gen Virol. 1990 Oct;71(Pt 10):2391–2399. doi: 10.1099/0022-1317-71-10-2391. [DOI] [PubMed] [Google Scholar]
- McCarthy A. M., McMahan L., Schaffer P. A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989 Jan;63(1):18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Michael N., Spector D., Mavromara-Nazos P., Kristie T. M., Roizman B. The DNA-binding properties of the major regulatory protein alpha 4 of herpes simplex viruses. Science. 1988 Mar 25;239(4847):1531–1534. doi: 10.1126/science.2832940. [DOI] [PubMed] [Google Scholar]
- Muller M. T. Binding of the herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J Virol. 1987 Mar;61(3):858–865. doi: 10.1128/jvi.61.3.858-865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J Virol. 1985 Dec;56(3):723–733. doi: 10.1128/jvi.56.3.723-733.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S. M., Sullivan-Tailyour G., Malone C. L., Stinski M. F. Subcellular localization of the major immediate early protein (IE1) of human cytomegalovirus at early times after infection. Virology. 1988 Feb;162(2):478–482. doi: 10.1016/0042-6822(88)90490-4. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Cross A., Haarr L., Orr A., Frame M. C., Murphy M., McGeoch D. J., Marsden H. S. Identification of the gene encoding the 65-kilodalton DNA-binding protein of herpes simplex virus type 1. J Virol. 1988 Mar;62(3):818–825. doi: 10.1128/jvi.62.3.818-825.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples M. E. Differential detergent treatment allows immunofluorescent localization of the Newcastle disease virus matrix protein within the nucleus of infected cells. Virology. 1988 Jan;162(1):255–259. doi: 10.1016/0042-6822(88)90418-7. [DOI] [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Koszinowski U. H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984 Nov 22;312(5992):369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987 Apr;61(4):1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W. R., Greene C. C., Aschman D. P., Schaffer P. A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985 Sep;55(3):796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A., Oroszlan S. Myristylation of gag-onc fusion proteins in mammalian transforming retroviruses. Virology. 1984 Mar;133(2):431–437. doi: 10.1016/0042-6822(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Sears A. E., Halliburton I. W., Meignier B., Silver S., Roizman B. Herpes simplex virus 1 mutant deleted in the alpha 22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985 Aug;55(2):338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. R., Butel J. S. Subcellular Localization of simian virus 40 large tumor antigen. J Virol. 1979 May;30(2):523–532. doi: 10.1128/jvi.30.2.523-532.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Knipe D. M. Herpes simplex virus alpha protein ICP27 can inhibit or augment viral gene transactivation. Virology. 1989 Jun;170(2):496–504. doi: 10.1016/0042-6822(89)90441-8. [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Kohn A., Sklyanskaya E., Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980 Jan;33(1):167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F., Courtney R. J. A major transcriptional regulatory protein (ICP4) of herpes simplex virus type 1 is associated with purified virions. J Virol. 1989 Aug;63(8):3338–3344. doi: 10.1128/jvi.63.8.3338-3344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]