Abstract
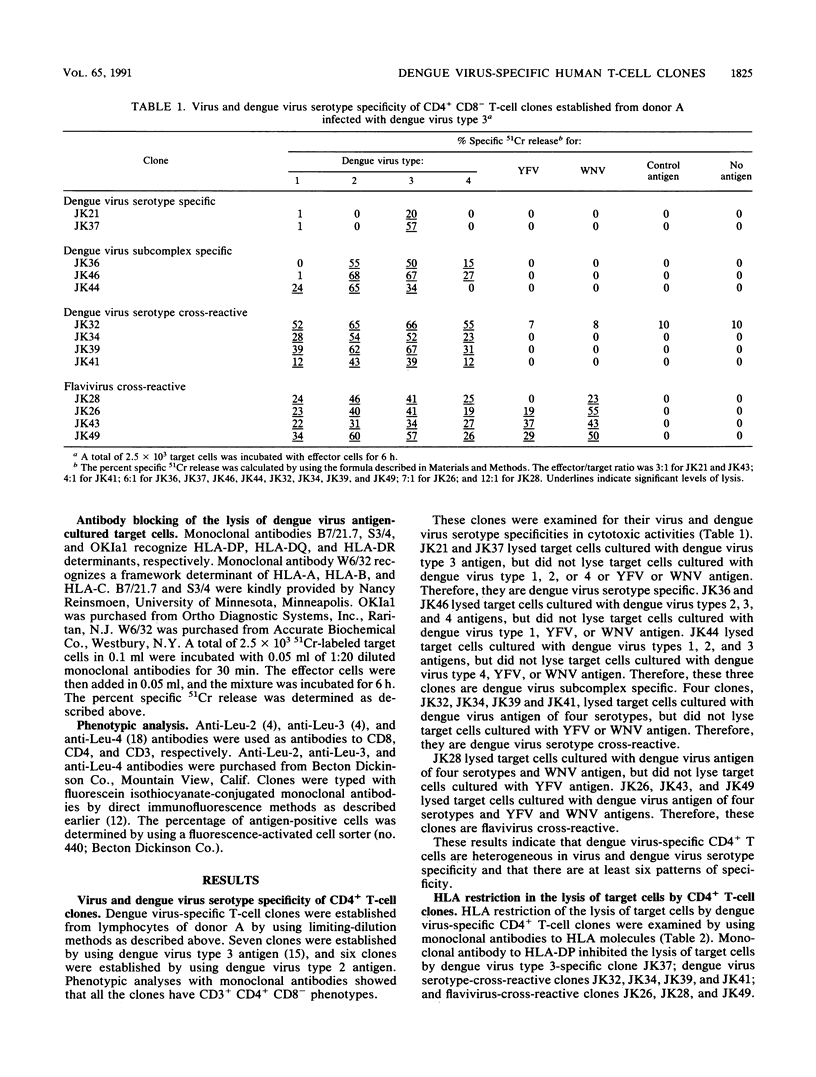
Thirteen dengue virus-specific, cytotoxic CD4+ CD8- T-cell clones were established from a donor who was infected with dengue virus type 3. These clones were examined for virus specificity and human leukocyte antigen (HLA) restriction in cytotoxic assays. Six patterns of virus specificities were determined. Two serotype-specific clones recognized only dengue virus type 3. Two dengue virus subcomplex-specific clones recognized dengue virus types 2, 3, and 4, and one subcomplex-specific clone recognized dengue virus types 1, 2, and 3. Four dengue virus serotype-cross-reactive clones recognized dengue virus types 1, 2, 3, and 4. One flavivirus-cross-reactive clone recognized dengue virus types 1, 2, 3, and 4 and West Nile virus (WNV), but did not recognize yellow fever virus (YFV), whereas three flavivirus-cross-reactive clones recognized dengue virus types 1, 2, 3, and 4, WNV, and YFV. HLA restriction in the lysis by these T-cell clones was also heterogeneous. HLA-DP, HLA-DQ, and HLA-DR were used as restriction elements by various T-cell clones. We also examined the recognition of viral nonstructural protein NS3, purified from cells infected with dengue virus type 3 or WNV, by these T-cell clones. One serotype-specific clone, two dengue virus subcomplex-specific clones, and three dengue virus serotype-cross-reactive clones recognized NS3 of dengue virus type 3. One flavivirus-cross-reactive clone recognized NS3 of dengue virus type 3 and WNV. These results indicate that heterogeneous dengue virus-specific CD4+ cytotoxic T cells are stimulated in response to infection with a dengue virus and that a nonstructural protein, NS3, contains multiple dominant T-cell epitopes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukowski J. F., Kurane I., Lai C. J., Bray M., Falgout B., Ennis F. A. Dengue virus-specific cross-reactive CD8+ human cytotoxic T lymphocytes. J Virol. 1989 Dec;63(12):5086–5091. doi: 10.1128/jvi.63.12.5086-5091.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. S., Nisalak A., Johnson D. E., Scott R. M. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988 Jan;38(1):172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Glickman E., Evans R. L. Antibodies to membrane structures that distinguish suppressor/cytotoxic and helper T lymphocyte subpopulations block the mixed leukocyte reaction in man. J Exp Med. 1981 Jul 1;154(1):193–198. doi: 10.1084/jem.154.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Koonin E. V., Blinov V. M. N-terminal domains of putative helicases of flavi- and pestiviruses may be serine proteases. Nucleic Acids Res. 1989 May 25;17(10):3889–3897. doi: 10.1093/nar/17.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. B., Brinton M. A. Dissociation of NS5 from cell fractions containing West Nile virus-specific polymerase activity. J Virol. 1987 Nov;61(11):3641–3644. doi: 10.1128/jvi.61.11.3641-3644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988 Jan 29;239(4839):476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- Innis B. L., Eckels K. H., Kraiselburd E., Dubois D. R., Meadors G. F., Gubler D. J., Burke D. S., Bancroft W. H. Virulence of a live dengue virus vaccine candidate: a possible new marker of dengue virus attenuation. J Infect Dis. 1988 Oct;158(4):876–880. doi: 10.1093/infdis/158.4.876. [DOI] [PubMed] [Google Scholar]
- Irie K., Mohan P. M., Sasaguri Y., Putnak R., Padmanabhan R. Sequence analysis of cloned dengue virus type 2 genome (New Guinea-C strain). Gene. 1989 Feb 20;75(2):197–211. doi: 10.1016/0378-1119(89)90266-7. [DOI] [PubMed] [Google Scholar]
- Kurane I., Hebblewaite D., Brandt W. E., Ennis F. A. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Virol. 1984 Oct;52(1):223–230. doi: 10.1128/jvi.52.1.223-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Hebblewaite D., Ennis F. A. Characterization with monoclonal antibodies of human lymphocytes active in natural killing and antibody-dependent cell-mediated cytotoxicity of dengue virus-infected cells. Immunology. 1986 Jul;58(3):429–436. [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Innis B. L., Nisalak A., Hoke C., Nimmannitya S., Meager A., Ennis F. A. Human T cell responses to dengue virus antigens. Proliferative responses and interferon gamma production. J Clin Invest. 1989 Feb;83(2):506–513. doi: 10.1172/JCI113911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Meager A., Ennis F. A. Dengue virus-specific human T cell clones. Serotype crossreactive proliferation, interferon gamma production, and cytotoxic activity. J Exp Med. 1989 Sep 1;170(3):763–775. doi: 10.1084/jem.170.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E., Makino Y., Zhao B. T., Zhang Y. M., Markoff L., Buckler-White A., Guiler M., Chanock R., Lai C. J. The nucleotide sequence of dengue type 4 virus: analysis of genes coding for nonstructural proteins. Virology. 1987 Aug;159(2):217–228. doi: 10.1016/0042-6822(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Preugschat F., Yao C. W., Strauss J. H. In vitro processing of dengue virus type 2 nonstructural proteins NS2A, NS2B, and NS3. J Virol. 1990 Sep;64(9):4364–4374. doi: 10.1128/jvi.64.9.4364-4374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Sekhon G. S., Kennett R., Bodmer W. F., Bodmer J. Permanent lymphoid lines from genetically marked lymphocytes: success with lymphocytes recovered from frozen storage. Tissue Antigens. 1976 Mar;7(3):165–172. doi: 10.1111/j.1399-0039.1976.tb01047.x. [DOI] [PubMed] [Google Scholar]


