Abstract
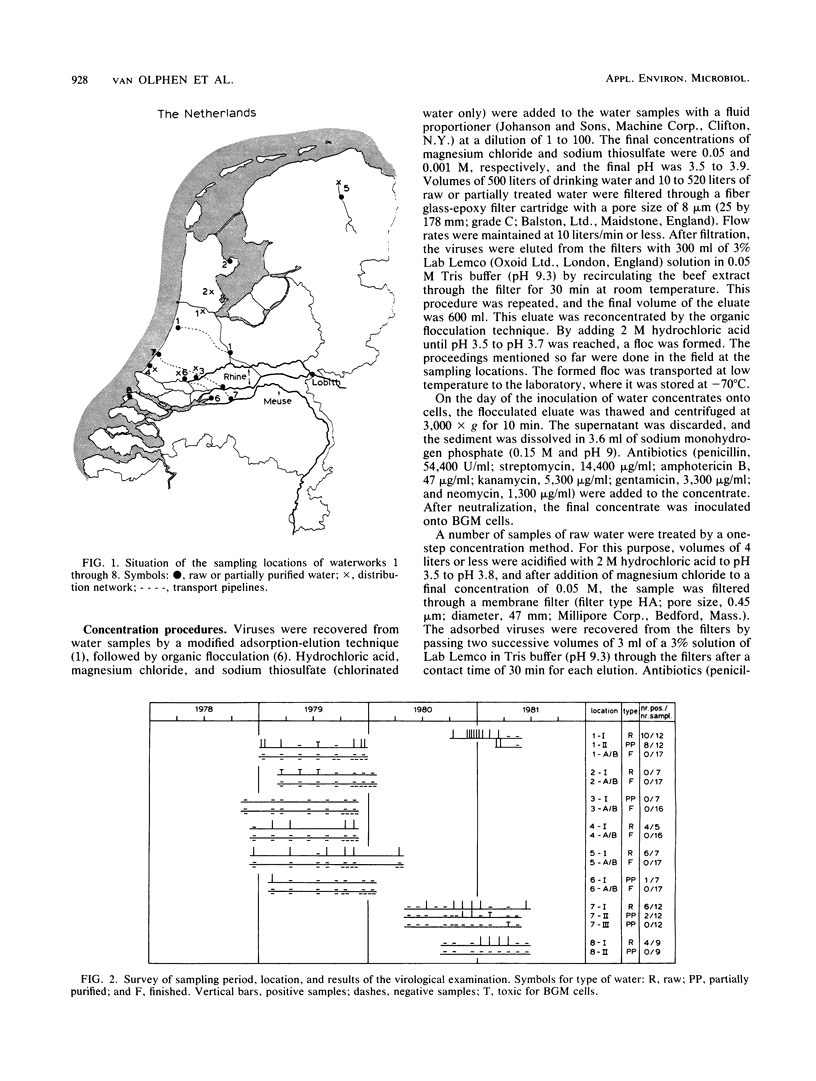
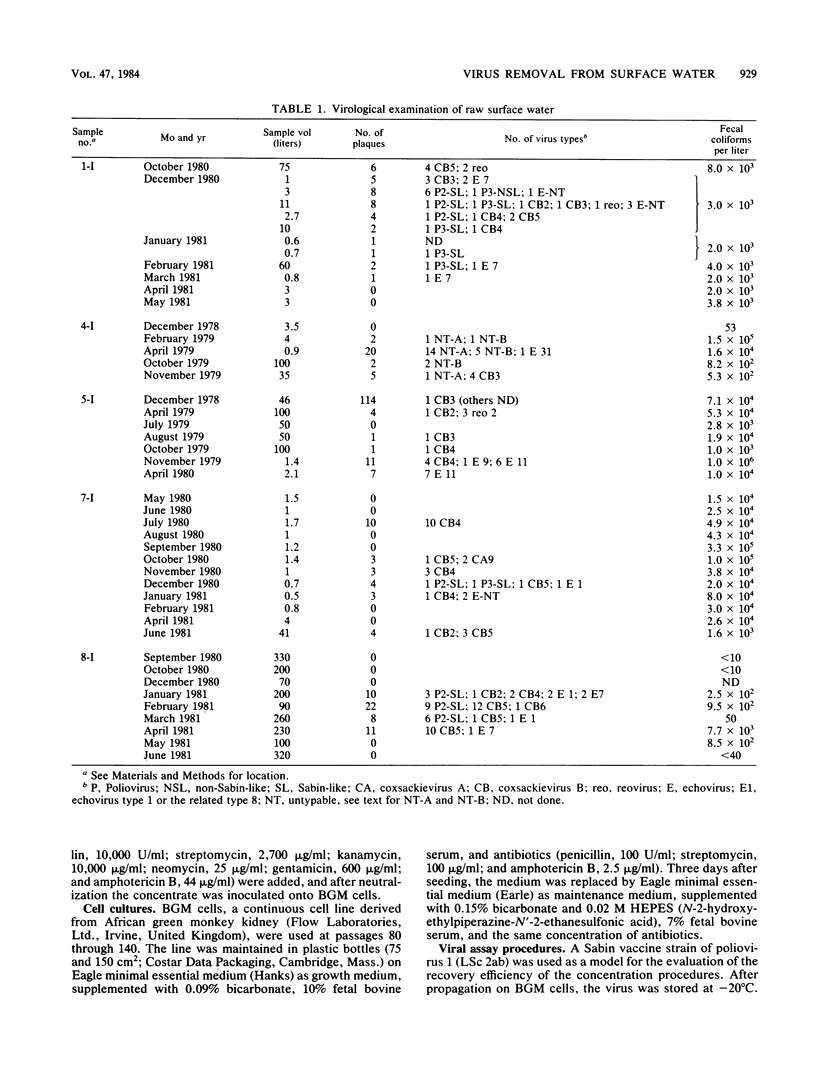
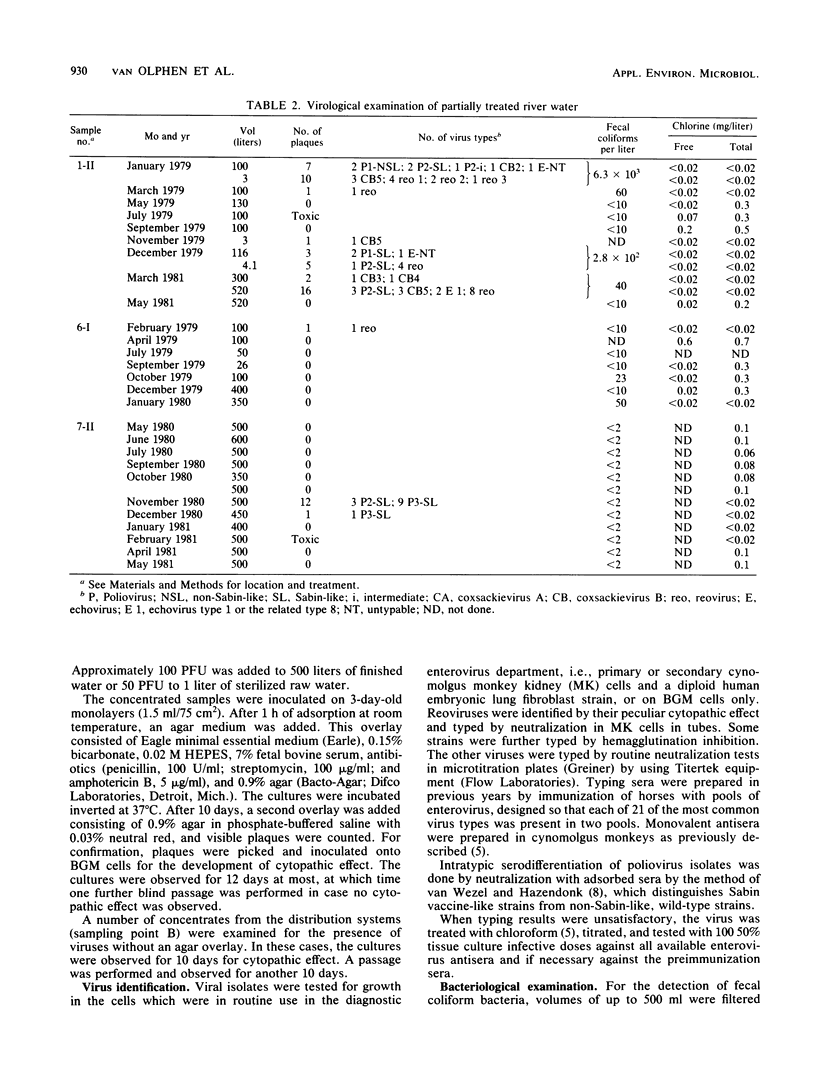
Eight waterworks in The Netherlands, which use surface water as their raw water source, were sampled repeatedly between November 1978 and June 1981. At five waterworks , 30 of 45 samples of raw water contained viruses. Of 55 samples of partially purified water, 11 were virus positive, including 8 after coagulation, sedimentation, and rapid sand filtration, 2 after storage, coagulation, sedimentation, transport chlorination, and rapid sand filtration, and 1 after storage in open reservoirs for 5 months. No viruses were detected in 100 samples of drinking water of 500 liters each from six waterworks . Most isolated viruses were typed, and a great variety of human enteroviruses were found, reflecting both pollution of raw water sources with sewage and vaccination with oral polio vaccine in neighboring countries.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hill W. F., Jr, Jakubowski W., Akin E. W., Clarke N. A. Detection of virus in water: sensitivity of the tentative standard method for drinking water. Appl Environ Microbiol. 1976 Feb;31(2):254–261. doi: 10.1128/aem.31.2.254-261.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving L. G., Smith F. A. One-year survey of enteroviruses, adenoviruses, and reoviruses isolated from effluent at an activated-sludge purification plant. Appl Environ Microbiol. 1981 Jan;41(1):51–59. doi: 10.1128/aem.41.1.51-59.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsenberg J. G., Brand-Saathof B. Jaaroverzicht 1980 van het diagnostische en epidemiologische virusonderzoek in Nederland. Ned Tijdschr Geneeskd. 1982 Mar 6;126(10):450–455. [PubMed] [Google Scholar]
- Kapsenberg J. G., Ras A., Korte J. Improvement of enterovirus neutralization by treatment with sodium deoxycholate or chloroform. Intervirology. 1980;12(6):329–334. doi: 10.1159/000149092. [DOI] [PubMed] [Google Scholar]
- Katzenelson E., Fattal B., Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976 Oct;32(4):638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus M. A., Hollick G. E. Increased Efficiency of Group B Coxsackievirus Isolation from Clinical Specimens by Use of BGM Cells. J Clin Microbiol. 1982 May;15(5):945–948. doi: 10.1128/jcm.15.5.945-948.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezel A. L., Hazendonk A. G. Intratypic serodifferentiation of poliomyelitis virus strains by strain-specific antisera. Intervirology. 1979;11(1):2–8. doi: 10.1159/000149005. [DOI] [PubMed] [Google Scholar]


