Abstract
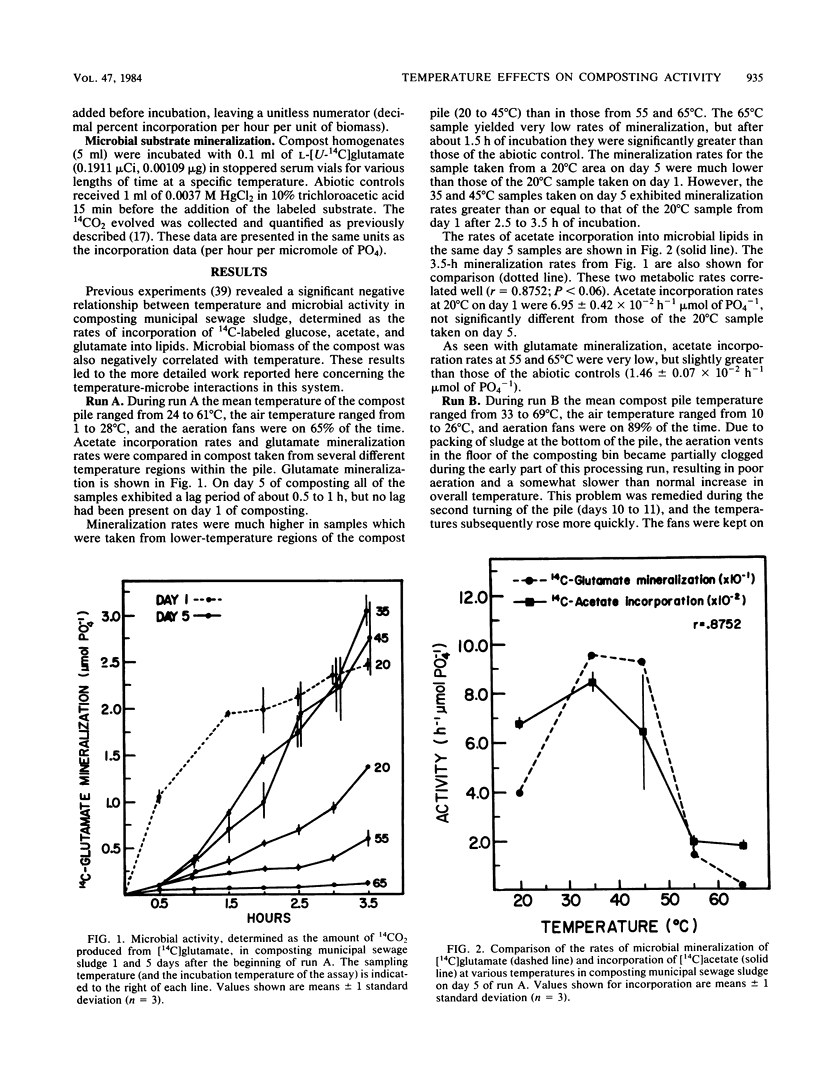
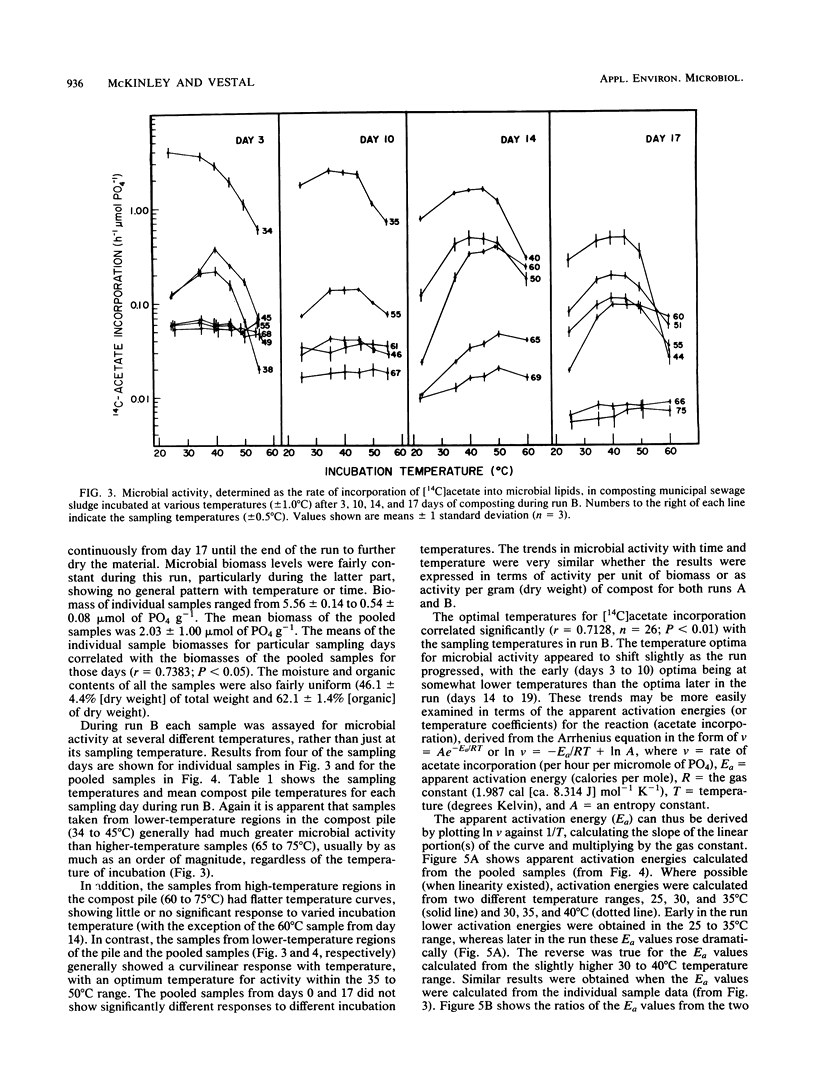
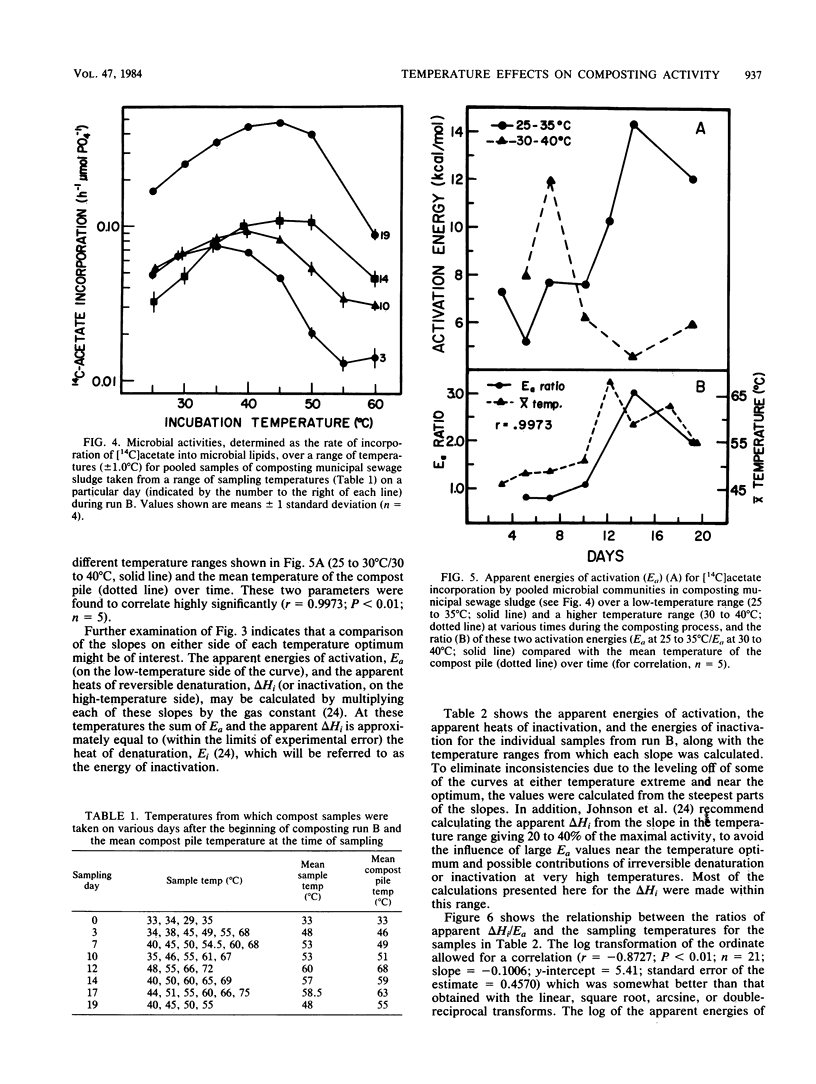
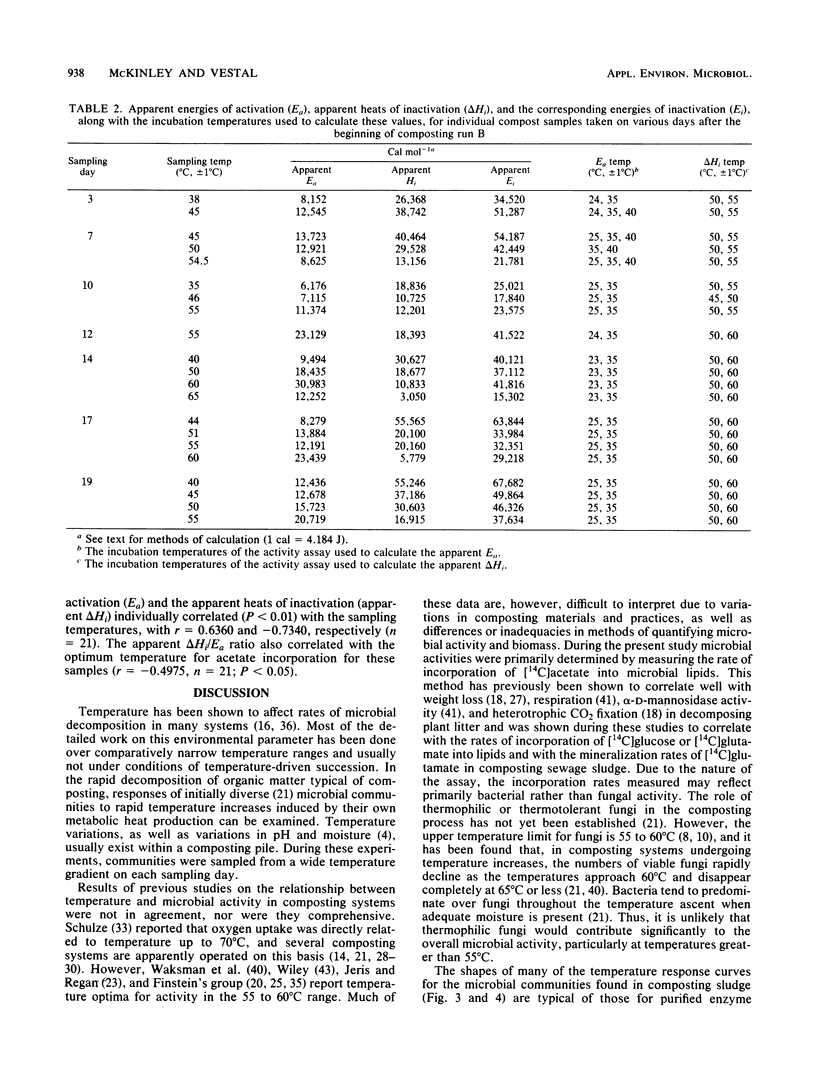
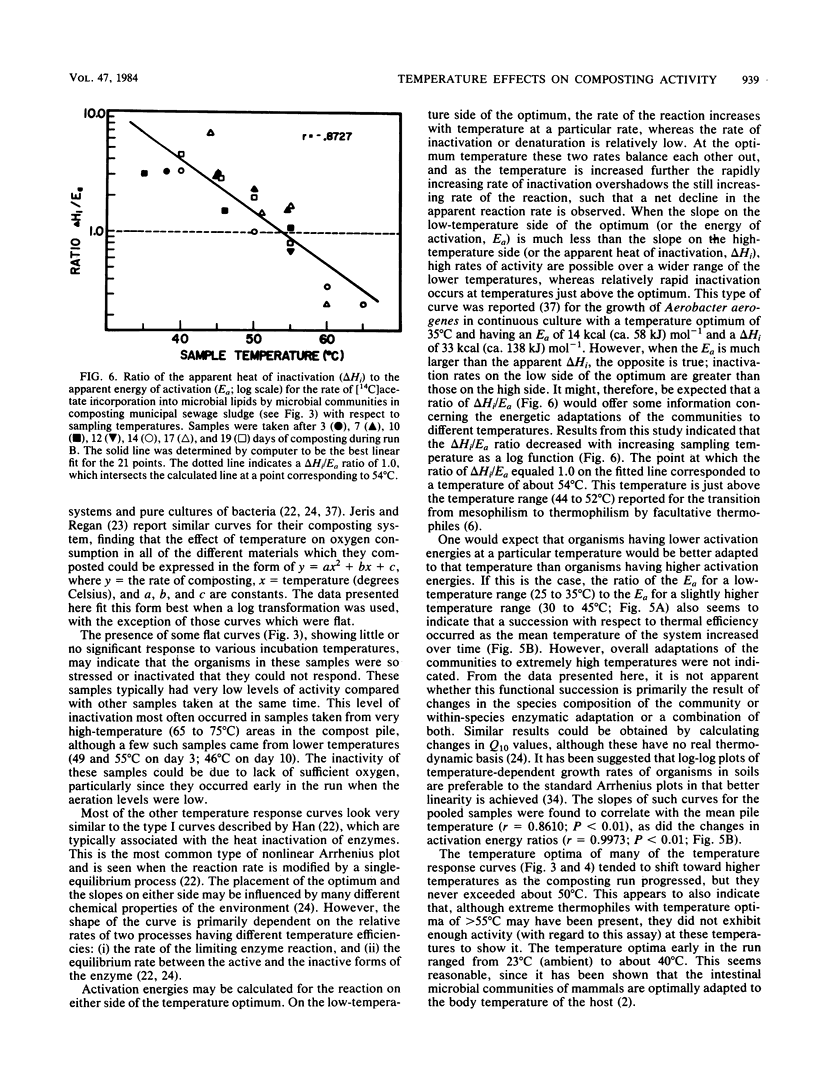
The interactions between temperature and the microbial communities in composting municipal sewage sludge were studied to determine the optimal temperature range for efficient decomposition (stabilization) of the sludge. Information concerning thermophilic successions in such communities was also obtained. Samples were taken from several different temperature areas in a production-scale composting pile throughout the 19-day processing run. Optimum temperatures for microbial activity, determined as the rate of [14C]acetate incorporation into microbial lipids, were determined for each sample. Biomass was determined from the lipid phosphate content of the sample. Maximal activities were generally found in samples coming from lower-temperature areas (25 to 45 degrees C), whereas samples from high temperatures (55 to 74 degrees C) usually had relatively little activity. The temperature giving the optimum activity in samples incubated at a variety of temperatures during the assay tended to increase as the composting time progressed, but never exceeded about 50 degrees C. Many of these temperature response curves were similar in nature to curves reported for purified enzyme systems and pure cultures of bacteria. Comparisons of the apparent energies of activation calculated for different temperature ranges over time also indicated that the overall community was better adapted to higher temperatures during the latter part of the composting run. It was also found that the relationship between the apparent energies of activation and the apparent energies of inactivation (apparent heats of denaturation) consistently changed with sample temperature throughout the composting run, suggesting that the microbial communities from hotter samples were better adapted to high temperatures than those from cooler samples, and vice versa.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. D., Brock T. D. The temperature optimum of the intestinal flora of the rat. Can J Microbiol. 1968 Jun;14(6):699–704. doi: 10.1139/m68-116. [DOI] [PubMed] [Google Scholar]
- Atchley S. H., Clark J. B. Variability of Temperature, pH, and Moisture in an Aerobic Composting Process. Appl Environ Microbiol. 1979 Dec;38(6):1040–1044. doi: 10.1128/aem.38.6.1040-1044.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Babel W., Rosenthal H. A., Rapoport S. A unified hypothesis on the causes of the cardinal temperatures of microorganisms; the temperature minimum of Bacillus stearothermophilus. Acta Biol Med Ger. 1972;28(4):565–576. [PubMed] [Google Scholar]
- Bausum H. T., Matney T. S. Boundary Between Bacterial Mesophilism and Thermophilism. J Bacteriol. 1965 Jul;90(1):50–53. doi: 10.1128/jb.90.1.50-53.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Brock T. D. Micro-organisms adapted to high temperatures. Nature. 1967 May 27;214(5091):882–885. doi: 10.1038/214882a0. [DOI] [PubMed] [Google Scholar]
- Federle T. W., Vestal J. R. Lignocellulose mineralization by arctic lake sediments in response to nutrient manipulation. Appl Environ Microbiol. 1980 Jul;40(1):32–39. doi: 10.1128/aem.40.1.32-39.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federle T. W., Vestal J. R. Microbial colonization and decomposition of carex litter in an arctic lake. Appl Environ Microbiol. 1980 Apr;39(4):888–893. doi: 10.1128/aem.39.4.888-893.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finstein M. S., Morris M. L. Microbiology of municipal solid waste composting. Adv Appl Microbiol. 1975;19:113–151. doi: 10.1016/s0065-2164(08)70427-1. [DOI] [PubMed] [Google Scholar]
- Han M. H. Non-linear Arrhenius plots in temperature-dependent kinetic studies of enzyme reactions. I. Single transition processes. J Theor Biol. 1972 Jun;35(3):543–568. doi: 10.1016/0022-5193(72)90150-6. [DOI] [PubMed] [Google Scholar]
- Macgregor S. T., Miller F. C., Psarianos K. M., Finstein M. S. Composting process control based on interaction between microbial heat output and temperature. Appl Environ Microbiol. 1981 Jun;41(6):1321–1330. doi: 10.1128/aem.41.6.1321-1330.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley V. L., Federle T. W., Vestal J. R. Effects of petroleum hydrocarbons on plant litter microbiota in an arctic lake. Appl Environ Microbiol. 1982 Jan;43(1):129–135. doi: 10.1128/aem.43.1.129-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley V. L., Vestal J. R. Effects of Acid on plant litter decomposition in an arctic lake. Appl Environ Microbiol. 1982 May;43(5):1188–1195. doi: 10.1128/aem.43.5.1188-1195.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZE K. L. Continuous thermophilic composting. Appl Microbiol. 1962 Mar;10:108–122. doi: 10.1128/am.10.2.108-122.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suler D. J., Finstein M. S. Effect of Temperature, Aeration, and Moisture on CO(2) Formation in Bench-Scale, Continuously Thermophilic Composting of Solid Waste. Appl Environ Microbiol. 1977 Feb;33(2):345–350. doi: 10.1128/aem.33.2.345-350.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topiwala H., Sinclair C. G. Temperature relationship in continous culture. Biotechnol Bioeng. 1971 Nov;13(6):795–813. doi: 10.1002/bit.260130606. [DOI] [PubMed] [Google Scholar]


