Abstract
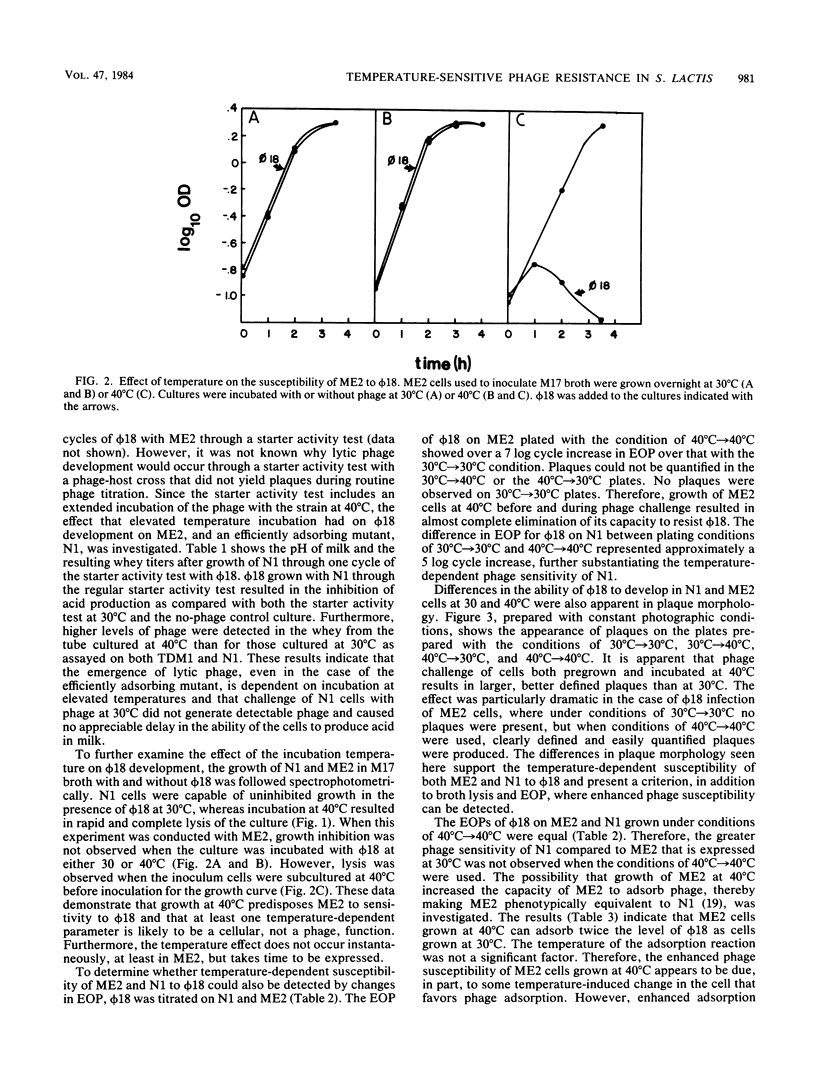
Streptococcus lactis ME2 is a dairy starter strain that is insensitive to a variety of phage, including φ18. The efficiency of plating of φ18 on ME2 and N1 could be increased from <1 × 10−9 to 5.0 × 10−2 and from 7.6 × 10−7 to 2.1 × 10−2, respectively, when the host strains were subcultured at 40°C before plating the phage and the phage assay plates were incubated at 40°C. Host-dependent replication was demonstrated in N1 at 30°C and in N1 and ME2 at 40°C, suggesting the operation of a temperature-sensitive restriction and modification system in ME2 and N1. The increased sensitivity of ME2 and N1 to φ18 at 40°C was also demonstrated by lysis of broth cultures and increased plaque size. ME2 grown at 40°C showed an increased ability to adsorb φ18, indicating a second target for temperature-dependent phage sensitivity in ME2. Challenge of N1 with a φ18 preparation that had been previously modified for growth on N1 indicated that at 40°C phage development was characterized by a shorter latent period and larger burst size than at 30°C. The evidence presented suggests that the high degree of phage insensitivity expressed by ME2 consists of a variety of temperature-sensitive mechanisms, including (i) the prevention of phage adsorption, (ii) host-controlled restriction of phage, and (iii) suppression of phage development. At 30°C these factors appear to act cooperatively to prevent the successful emergence of lytic phage active against S. lactis ME2.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W. DNA modification and restriction. Prog Nucleic Acid Res Mol Biol. 1974;14(0):1–37. doi: 10.1016/s0079-6603(08)60204-4. [DOI] [PubMed] [Google Scholar]
- COLLINS E. B. Host-controlled variations in bacteriophages active against lactic streptococci. Virology. 1956 Apr;2(2):261–271. doi: 10.1016/0042-6822(56)90021-6. [DOI] [PubMed] [Google Scholar]
- Chopin M. C., Chopin A., Roux C. Definition of bacteriophage groups according to their lytic action on mesophilic lactic streptococci. Appl Environ Microbiol. 1976 Dec;32(6):741–746. doi: 10.1128/aem.32.6.741-746.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger D. H., Hansen S., Schroeder C. Host-dependent modification of bacterial virus T3 affecting its adsorption ability. Virology. 1980 Apr 30;102(2):444–446. doi: 10.1016/0042-6822(80)90111-7. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Hansen S., Schroeder C., Presber W. Host-dependent modification of bacteriophage T7 and SAMase-negative T3 derivatives affecting their adsorption ability. Mol Gen Genet. 1977 May 20;153(1):107–110. doi: 10.1007/BF01036002. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Schroeder C., Hansen S., Rosenthal H. A. Active protection by bacteriophages T3 and T7 against E. coli B- and K-specific restriction of their DNA. Mol Gen Genet. 1977 May 20;153(1):99–106. doi: 10.1007/BF01036001. [DOI] [PubMed] [Google Scholar]
- Potter N. N. Host-induced changes in lactic streptococcal bacteriophages. J Dairy Sci. 1970 Oct;53(10):1358–1362. doi: 10.3168/jds.S0022-0302(70)86398-6. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Characterization of Phage-Sensitive Mutants from a Phage-Insensitive Strain of Streptococcus lactis: Evidence for a Plasmid Determinant that Prevents Phage Adsorption. Appl Environ Microbiol. 1983 Nov;46(5):1125–1133. doi: 10.1128/aem.46.5.1125-1133.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Evidence for Plasmid Linkage of Restriction and Modification in Streptococcus cremoris KH. Appl Environ Microbiol. 1981 Dec;42(6):944–950. doi: 10.1128/aem.42.6.944-950.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Restriction and modification in group N streptococci: effect of heat on development of modified lytic bacteriophage. Appl Environ Microbiol. 1980 Sep;40(3):500–506. doi: 10.1128/aem.40.3.500-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



