Abstract
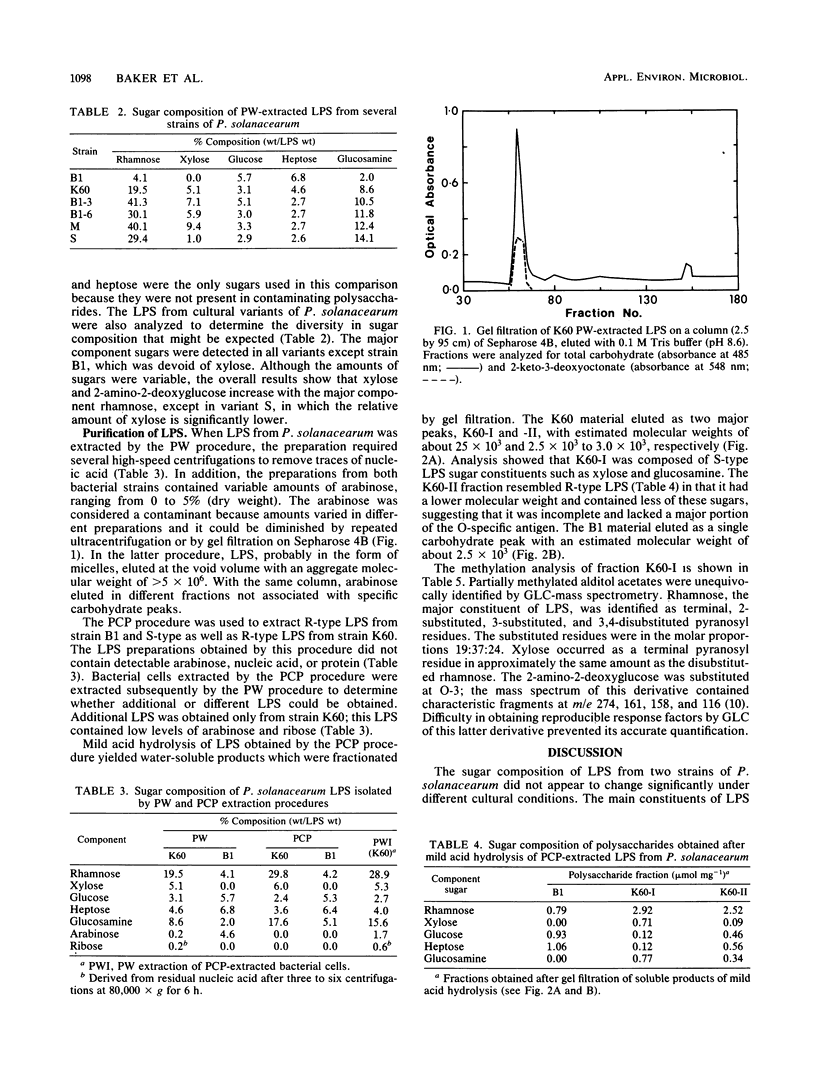
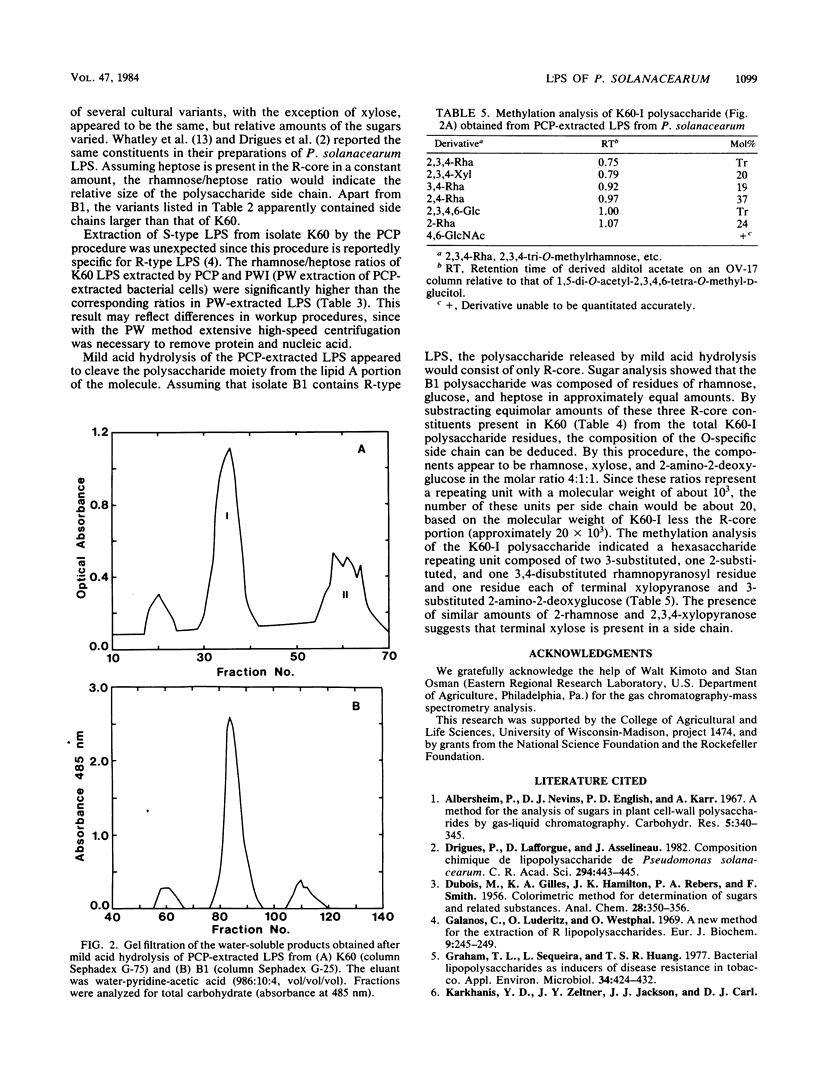
The carbohydrates present in lipopolysaccharide (LPS) from Pseudomonas solanacearum are rhamnose, xylose, 2-amino-2-deoxyglucose, glucose, heptose, and 2-keto-3-deoxyoctonate. LPS extracted from cultures grown on either glycerol or glucose (as the major source of carbon) and extracted after various incubation periods had similar compositions. The LPS from several strains of the bacterium contained the same component sugars, but the amounts of each sugar varied considerably. It was observed, however, that xylose and 2-amino-2-deoxyglucose increased proportionately with rhamnose, the major component. Phenol-water-extracted LPS contained measurable amounts of nucleic acid, protein, and arabinan, but none of these polymers were detected in LPS extracted with phenol-chloroform-petroleum ether. Polysaccharides liberated from LPS by mild acid hydrolysis were purified by gel filtration. Carbohydrate analysis of the LPS from a virulent, fluidal strain (K60) showed that the O-specific antigen consisted of rhamnose, xylose, and 2-amino-2-deoxyglucose in the proportions 4:1:1. The LPS of an avirulent, afluidal strain (B1) lacked the O-specific antigen; the R-core region consisted of rhamnose, glucose, heptose, and 2-keto-3-deoxyoctonate. Methylation analysis indicated that the K60 O-specific antigen was composed of a hexasaccharide repeating unit containing 3-, 2-, and 3,4-substituted rhamnopyranosyl residues, 3-substituted 2-amino-2-deoxyglucose, and terminal xylopyranose in the molar ratios 2:1:1:1:1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drigues P., Lafforgue D., Asselineau J. Composition chimique du lipopolysaccharide de Pseudomonas solanacearum. C R Seances Acad Sci III. 1982 Mar 15;294(11):443–445. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Graham T. L., Sequeira L., Huang T. S. Bacterial lipopolysaccharides as inducers of disease resistance in tobacco. Appl Environ Microbiol. 1977 Oct;34(4):424–432. doi: 10.1128/aem.34.4.424-432.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford P. A., Conrad H. E. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. I. A reexamination using improved procedures for methylation analysis. Biochemistry. 1966 May;5(5):1508–1517. doi: 10.1021/bi00869a009. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Different core regions in the lipopolysaccharides of O group 8. Eur J Biochem. 1969 Oct;10(3):501–510. doi: 10.1111/j.1432-1033.1969.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Stellner K., Saito H., Hakomori S. I. Determination of aminosugar linkages in glycolipids by methylation. Aminosugar linkages of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch Biochem Biophys. 1973 Apr;155(2):464–472. doi: 10.1016/0003-9861(73)90138-0. [DOI] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley M. H., Hunter N., Cantrell M. A., Hendrick C., Keegstra K., Sequeira L. Lipopolysaccharide Composition of the Wilt Pathogen, Pseudomonas solanacearum: CORRELATION WITH THE HYPERSENSITIVE RESPONSE IN TOBACCO. Plant Physiol. 1980 Mar;65(3):557–559. doi: 10.1104/pp.65.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]


