Abstract
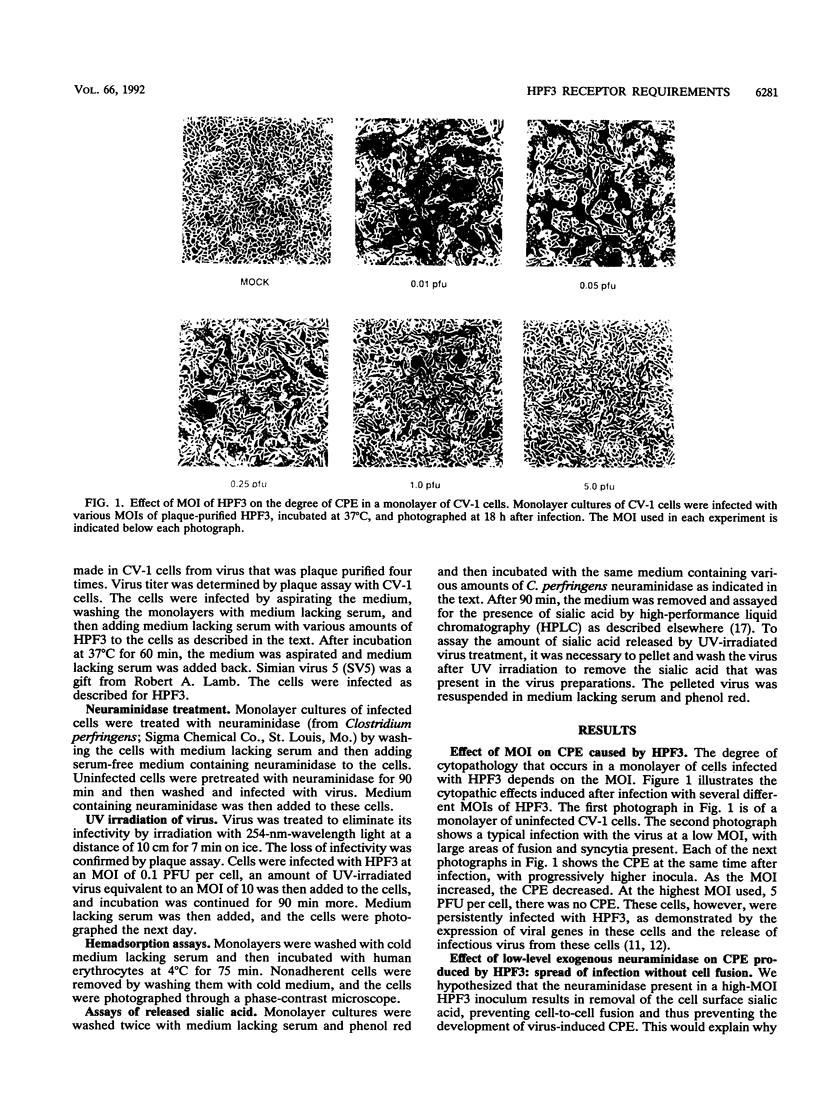
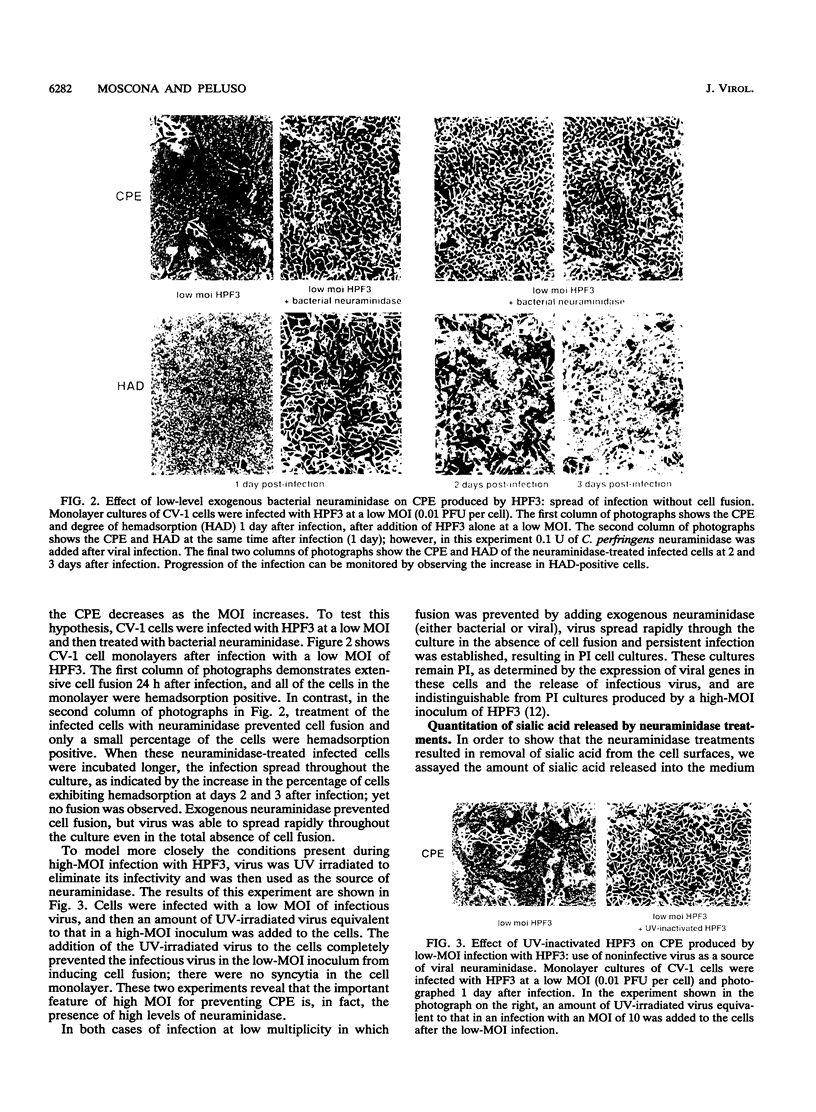
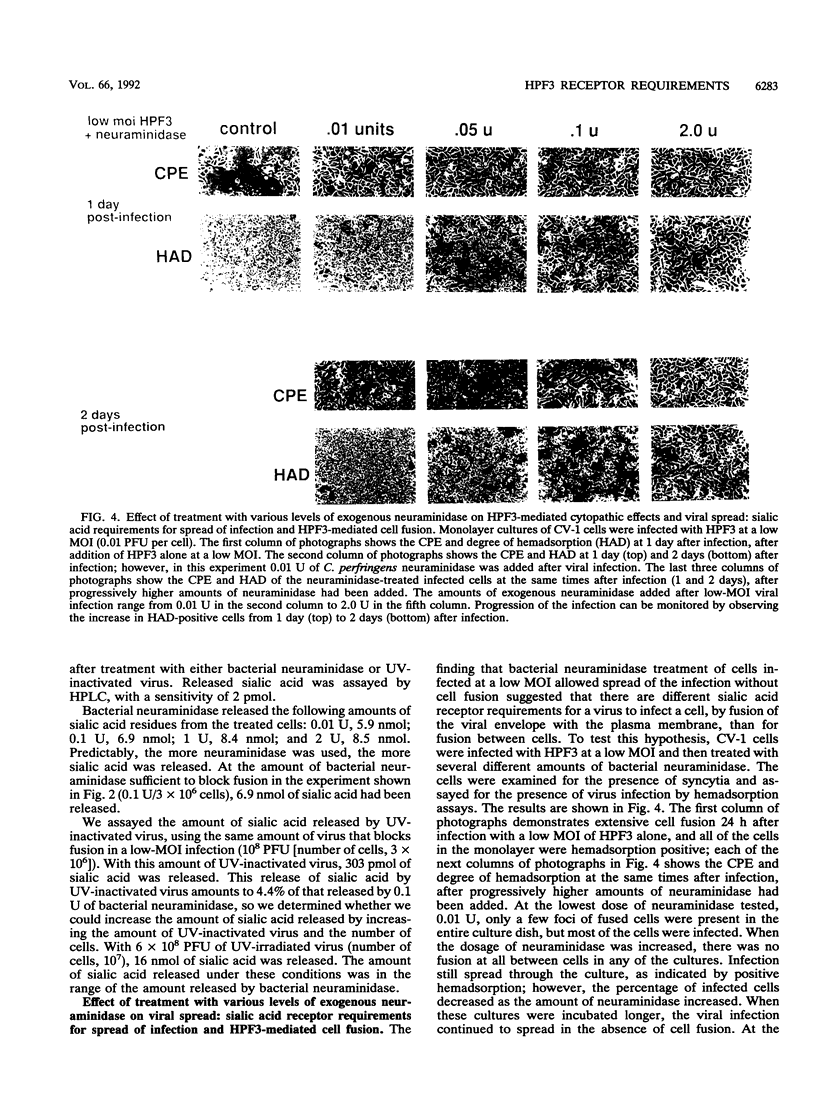
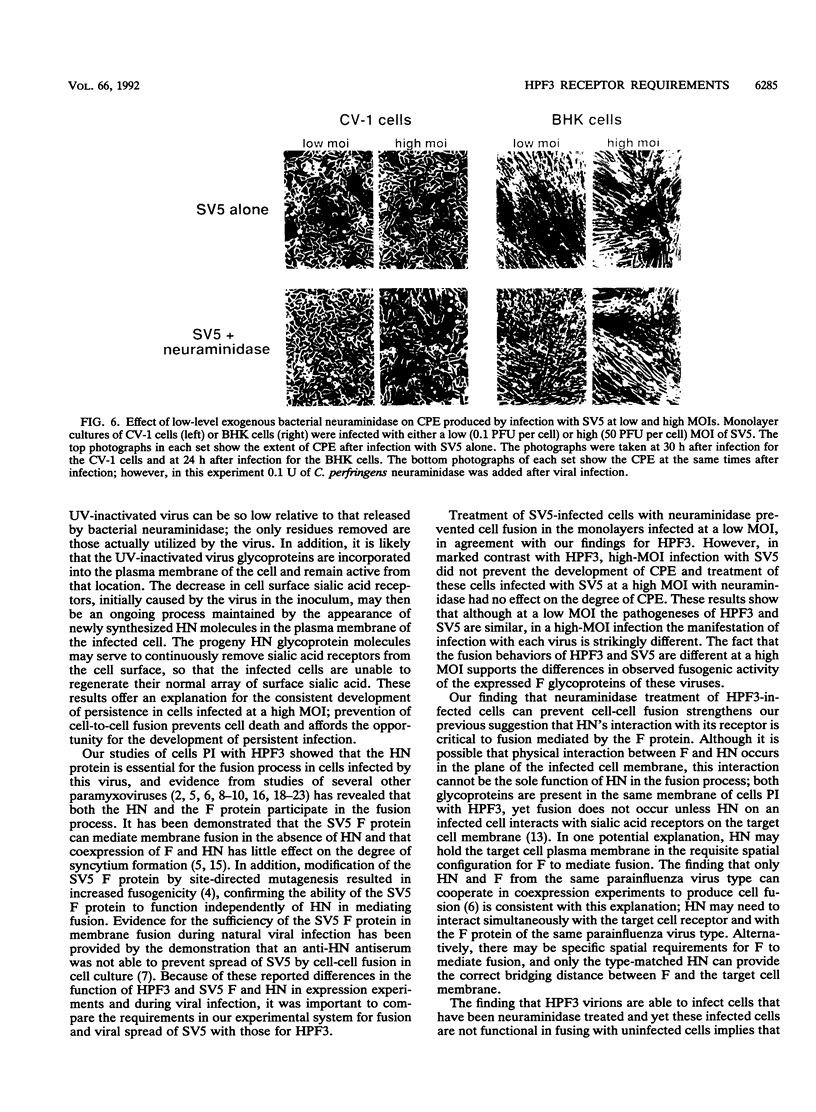
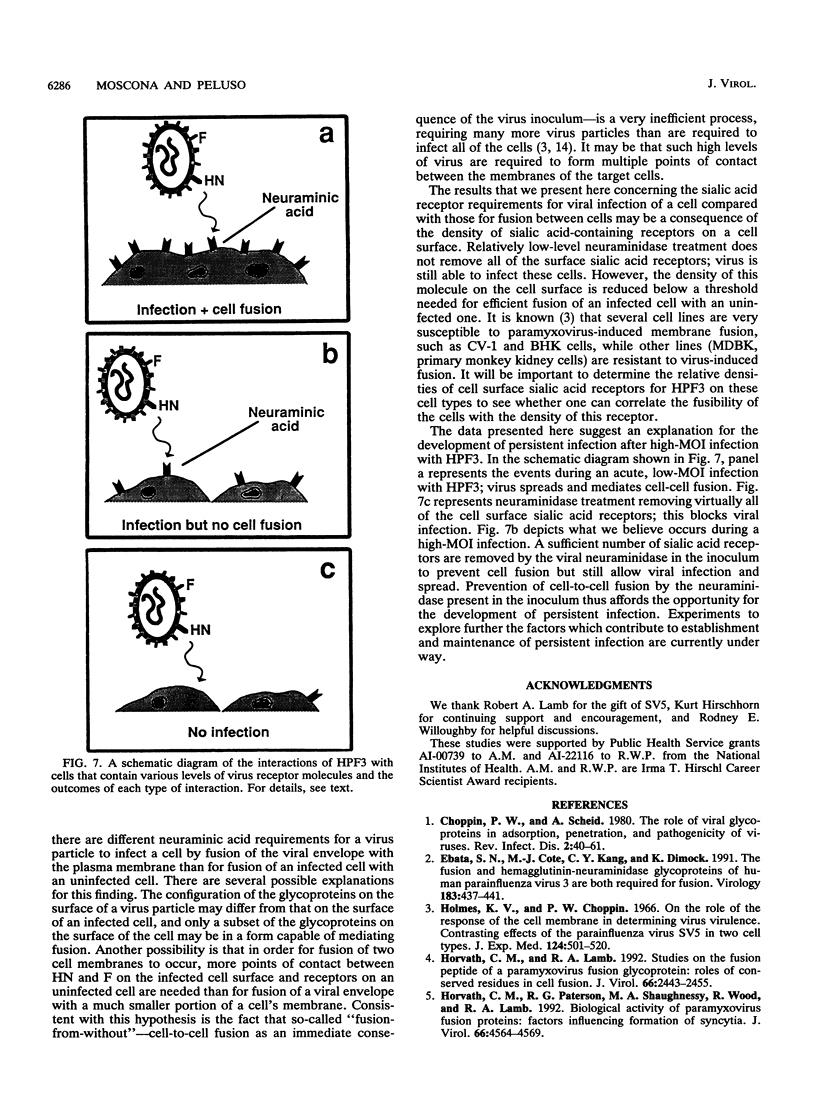
Cells can be persistently infected with human parainfluenza virus type 3 (HPF3) by using a high multiplicity of infection (MOI) (> or = 5 PFU per cell). The persistently infected cells exhibit no cytopathic effects and do not fuse with each other, yet they readily fuse with uninfected cells. We have previously shown that the failure of the persistently infected cells to fuse with each other is due to the lack of a receptor on these cells for the viral hemagglutinin-neuraminidase glycoprotein, and we have established that both fusion and hemagglutinin-neuraminidase proteins are needed for cell fusion mediated by HPF3. We then postulated that the generation of persistent infection and the failure of cells infected with HPF3 at high MOI to form syncytia are both due to the action of viral neuraminidase in the high-MOI inoculum. In this report, we describe experiments to test this hypothesis and further investigate the receptor requirements for HPF3 infection and cell fusion. A normally cytopathic low-MOI HPF3 infection can be converted into a noncytopathic infection by the addition of exogenous neuraminidase, either in the form of a purified enzyme or as UV-inactivated HPF3 virions. Evidence is presented that the receptor requirements for an HPF3 virus particle to infect a cell are different from those for fusion between cells. By treating infected cells in culture with various doses of neuraminidase, we demonstrate that virus spreads from cell to cell in the complete absence of cell-cell fusion. We compare the outcome of HPF3 infection in the presence of excess neuraminidase with that of another paramyxovirus (simian virus 5) and provide evidence that these two viruses differ in their receptor requirements for mediating fusion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Ebata S. N., Côté M. J., Kang C. Y., Dimock K. The fusion and hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus 3 are both required for fusion. Virology. 1991 Jul;183(1):437–441. doi: 10.1016/0042-6822(91)90162-5. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C. M., Lamb R. A. Studies on the fusion peptide of a paramyxovirus fusion glycoprotein: roles of conserved residues in cell fusion. J Virol. 1992 Apr;66(4):2443–2455. doi: 10.1128/jvi.66.4.2443-2455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C. M., Paterson R. G., Shaughnessy M. A., Wood R., Lamb R. A. Biological activity of paramyxovirus fusion proteins: factors influencing formation of syncytia. J Virol. 1992 Jul;66(7):4564–4569. doi: 10.1128/jvi.66.7.4564-4569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. L., Ray R., Compans R. W. Functional interactions between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J Virol. 1992 Mar;66(3):1528–1534. doi: 10.1128/jvi.66.3.1528-1534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Wolinsky J. S. Biochemical features of mumps virus neuraminidases and their relationship with pathogenicity. Virology. 1981 Oct 15;114(1):218–227. doi: 10.1016/0042-6822(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Wolinsky J. S. Conversion of nonfusing mumps virus infections to fusing infections by selective proteolysis of the HN glycoprotein. Virology. 1983 Dec;131(2):328–340. doi: 10.1016/0042-6822(83)90501-9. [DOI] [PubMed] [Google Scholar]
- Morrison T., McQuain C., McGinnes L. Complementation between avirulent Newcastle disease virus and a fusion protein gene expressed from a retrovirus vector: requirements for membrane fusion. J Virol. 1991 Feb;65(2):813–822. doi: 10.1128/jvi.65.2.813-822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. Defective interfering particles of human parainfluenza virus type 3 are associated with persistent infection in cell culture. Virology. 1991 Aug;183(2):821–824. doi: 10.1016/0042-6822(91)91018-c. [DOI] [PubMed] [Google Scholar]
- Moscona A., Galinski M. S. Characterization of human parainfluenza virus type 3 persistent infection in cell culture. J Virol. 1990 Jul;64(7):3212–3218. doi: 10.1128/jvi.64.7.3212-3218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Peluso R. W. Fusion properties of cells persistently infected with human parainfluenza virus type 3: participation of hemagglutinin-neuraminidase in membrane fusion. J Virol. 1991 Jun;65(6):2773–2777. doi: 10.1128/jvi.65.6.2773-2777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. I. Microscopic observation of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:98–107. doi: 10.1016/0014-4827(62)90205-7. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Hiebert S. W., Lamb R. A. Expression at the cell surface of biologically active fusion and hemagglutinin/neuraminidase proteins of the paramyxovirus simian virus 5 from cloned cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Metzger D. W. Distinct functions of antigenic sites of the HN glycoprotein of Sendai virus. Virology. 1987 May;158(1):61–68. doi: 10.1016/0042-6822(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Powell L. D., Hart G. W. Quantitation of picomole levels of N-acetyl- and N-glycolylneuraminic acids by a HPLC-adaptation of the thiobarbituric acid assay. Anal Biochem. 1986 Aug 15;157(1):179–185. doi: 10.1016/0003-2697(86)90211-3. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Shibuta H. Syncytium formation by recombinant vaccinia viruses carrying bovine parainfluenza 3 virus envelope protein genes. J Virol. 1989 Sep;63(9):3661–3668. doi: 10.1128/jvi.63.9.3661-3668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuta H., Kanda T., Hazama A., Adachi A., Matumoto M. Parainfluenza 3 virus: plaque-type variants lacking neuraminidase activity. Infect Immun. 1981 Oct;34(1):262–267. doi: 10.1128/iai.34.1.262-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuta H., Nozawa A., Shioda T., Kanda T. Neuraminidase activity and syncytial formation in variants of parainfluenza 3 virus. Infect Immun. 1983 Aug;41(2):780–788. doi: 10.1128/iai.41.2.780-788.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurudome M., Yamada A., Hishiyama M., Ito Y. Monoclonal antibodies against the glycoproteins of mumps virus: fusion inhibition by anti-HN monoclonal antibody. J Gen Virol. 1986 Oct;67(Pt 10):2259–2265. doi: 10.1099/0022-1317-67-10-2259. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J. Identification of amino acids involved in the sialidase activity of the mumps virus hemagglutinin-neuraminadase protein. Virology. 1988 Nov;167(1):226–232. doi: 10.1016/0042-6822(88)90072-4. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. A fusing mumps virus variant selected from a nonfusing parent with the neuraminidase inhibitor 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1986 Jun;151(2):286–295. doi: 10.1016/0042-6822(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Lambert D. M., Galinski M. S., Mink M. A., Rochovansky O., Pons M. W. Immediate persistent infection by human parainfluenza virus 3: unique fusion properties of the persistently infected cells. J Gen Virol. 1987 Jun;68(Pt 6):1737–1748. doi: 10.1099/0022-1317-68-6-1737. [DOI] [PubMed] [Google Scholar]








