Abstract
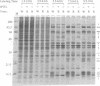
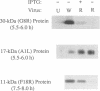
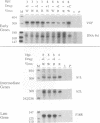
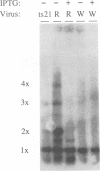
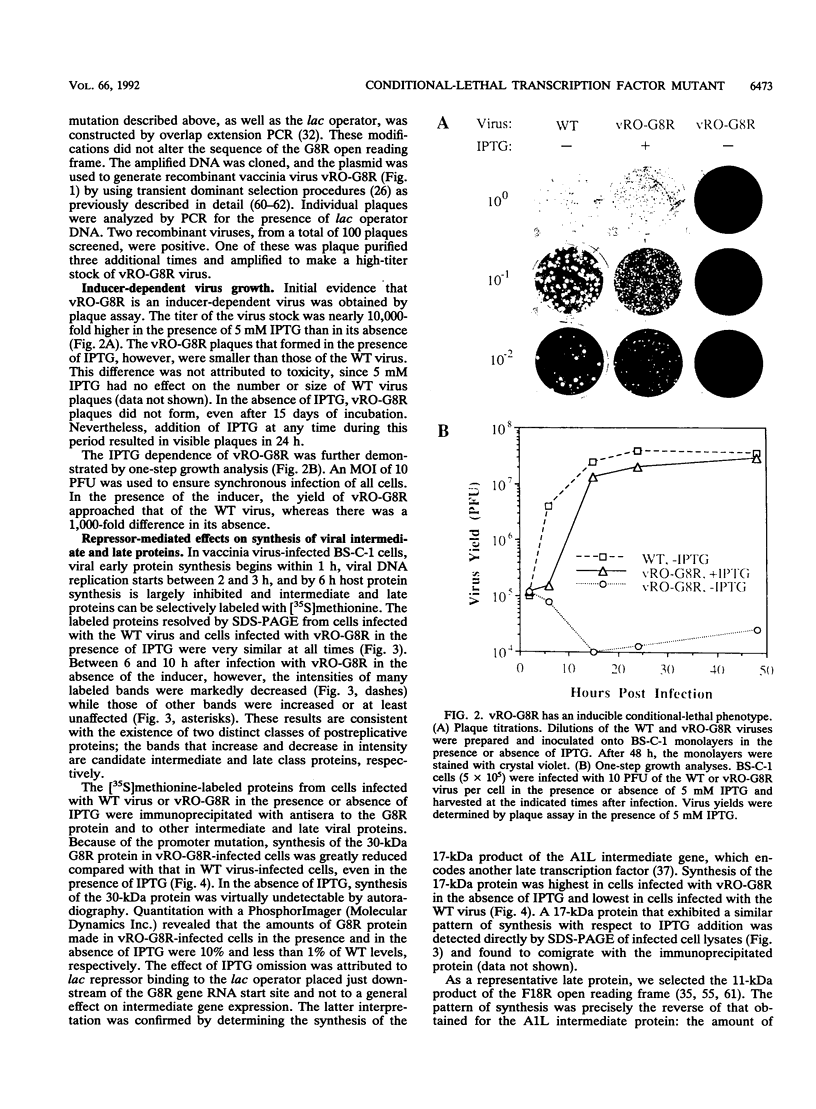
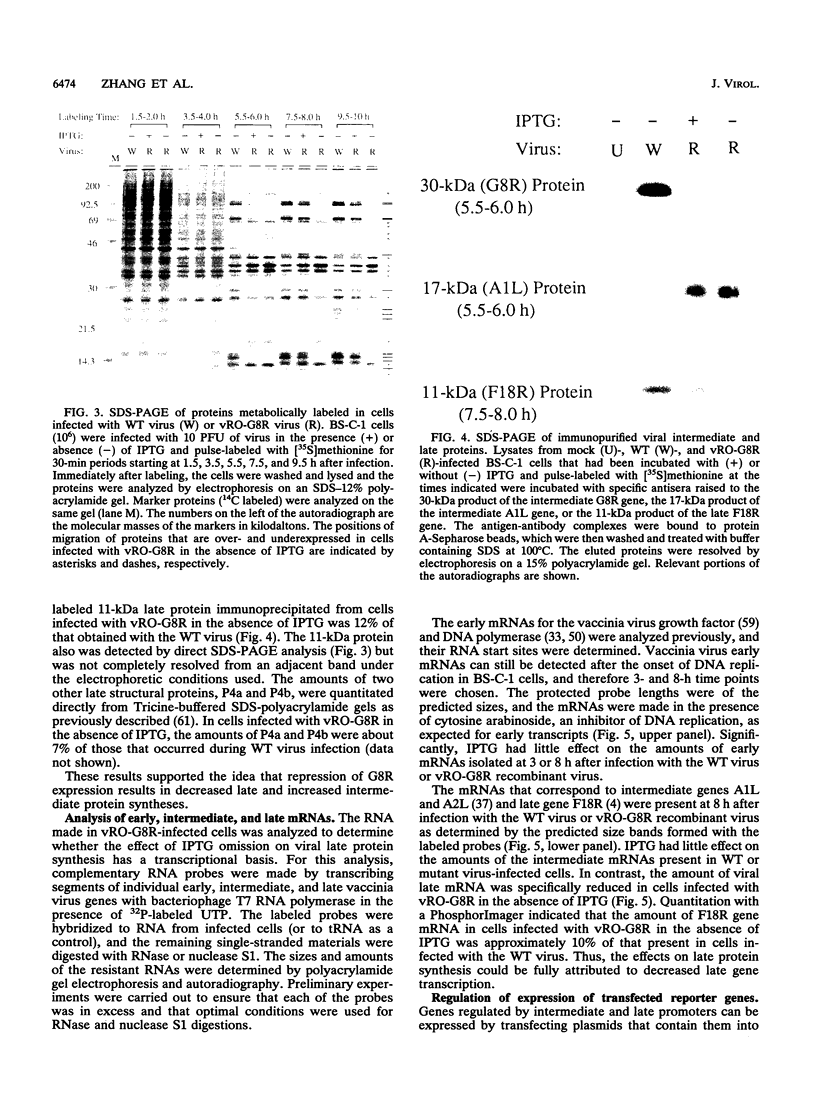
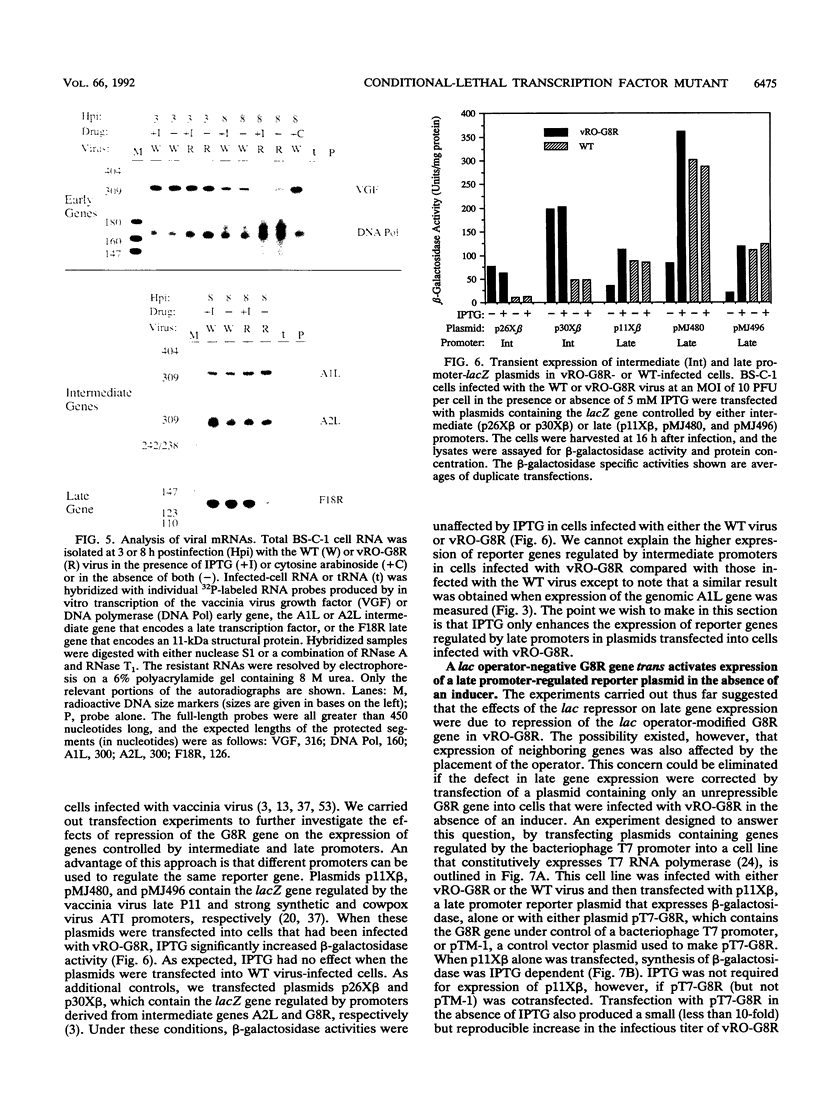
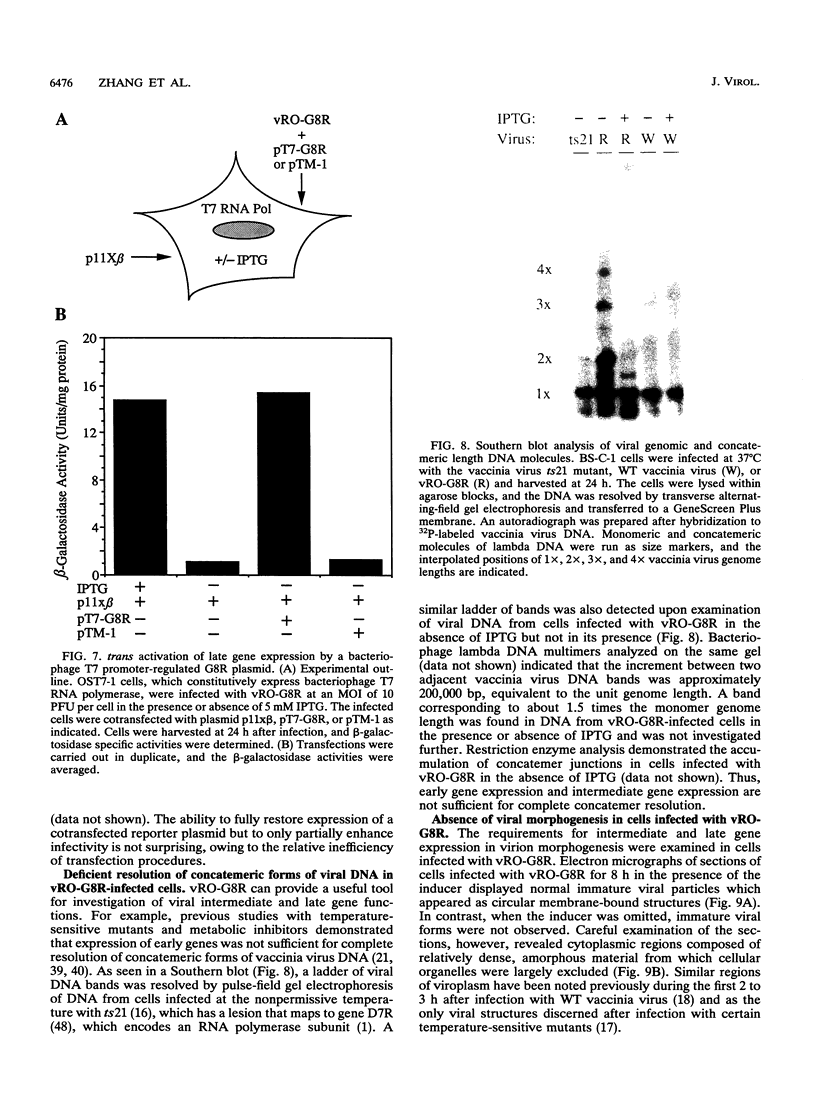
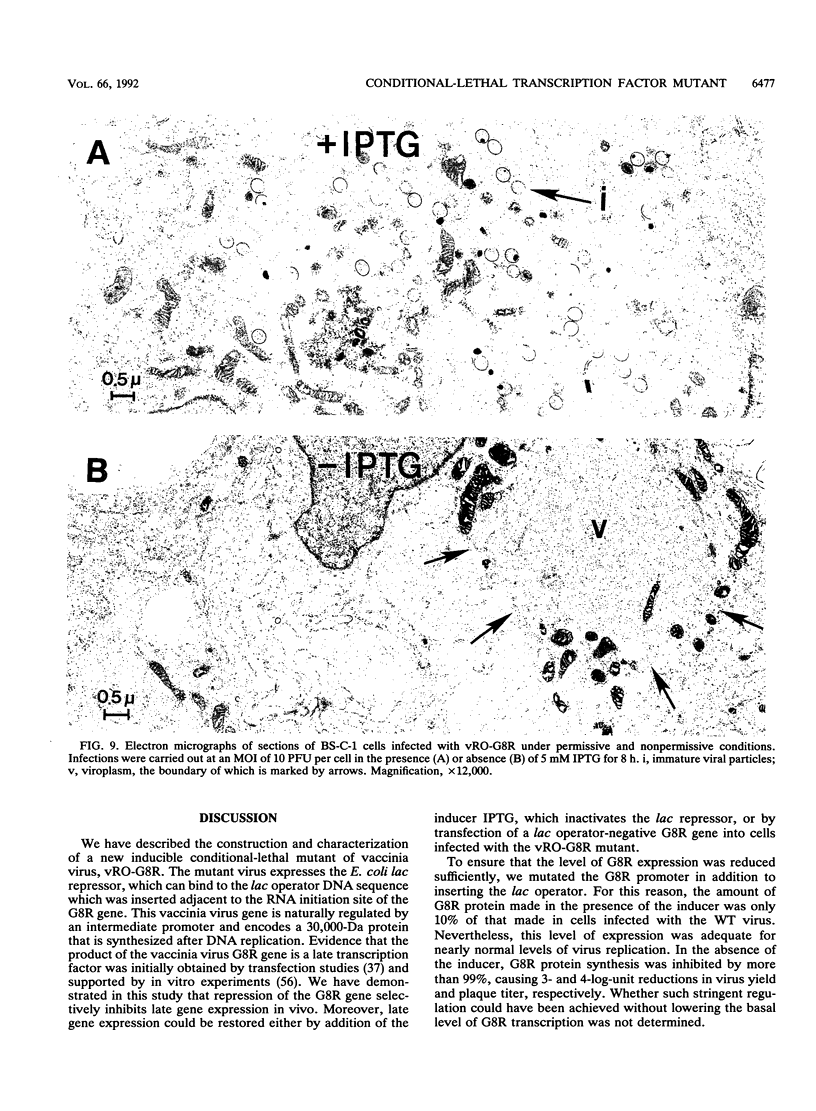
There are three temporal classes of vaccinia virus genes: early, intermediate, and late. The object of this study was to determine the effects on virus replication of regulating the expression of G8R, an intermediate gene that encodes a late transcription factor. We inserted the lac operator adjacent to the RNA start site of the G8R gene in a recombinant vaccinia virus that constitutively expresses the Escherichia coli lac repressor to make expression of the G8R gene dependent on the inducer isopropyl-beta-D-thiogalactopyranoside (IPTG). In case repression would not be complete, we also weakened the promoter of the G8R gene by making a single-nucleotide substitution designed to reduce its basal level of transcription. The mutant virus replicated well in the presence of the inducer, although synthesis of the G8R-encoded 30,000-M(r) protein was only 10% of that of the wild-type virus. In the absence of IPTG, (i) synthesis of the G8R protein was inhibited by more than 99% relative to that of the wild-type virus, (ii) synthesis of early and intermediate mRNAs appeared to be unaffected, (iii) intermediate proteins accumulated to higher than normal levels, (iv) synthesis of late mRNA and protein was reduced by about 90%, (v) viral DNA was replicated but incompletely resolved concatemeric molecules accumulated, (vi) not even the earliest stages of virion assembly were detectable by transmission electron microscopy, and (vii) virus yield under one-step growth conditions and plaque formation were 10(-3) and 10(-4) times the wild-type values, respectively. The defect in late gene expression could be overcome by transfection of a G8R gene that was not under lac operator control, as well as by addition of IPTG, further demonstrating the specificity of the repression. The correlation between decreased expression of the G8R intermediate gene and inhibition of late mRNA synthesis is consistent with the notion that the G8R product serves as an essential late transcription factor and supports a cascade mechanism of vaccinia virus gene regulation. In addition, the inducer-dependent vaccinia virus mutant provided a tool for selective inhibition of late gene expression while allowing synthesis of early and intermediate mRNAs and proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn B. Y., Jones E. V., Moss B. Identification of the vaccinia virus gene encoding an 18-kilodalton subunit of RNA polymerase and demonstration of a 5' poly(A) leader on its early transcript. J Virol. 1990 Jun;64(6):3019–3024. doi: 10.1128/jvi.64.6.3019-3024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B. Y., Moss B. RNA polymerase-associated transcription specificity factor encoded by vaccinia virus. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3536–3540. doi: 10.1073/pnas.89.8.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldick C. J., Jr, Keck J. G., Moss B. Mutational analysis of the core, spacer, and initiator regions of vaccinia virus intermediate-class promoters. J Virol. 1992 Aug;66(8):4710–4719. doi: 10.1128/jvi.66.8.4710-4719.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet C., Drillien R., Wittek R. One hundred base pairs of 5' flanking sequence of a vaccinia virus late gene are sufficient to temporally regulate late transcription. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2096–2100. doi: 10.1073/pnas.82.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S. A role for ATP hydrolysis in vaccinia virus early gene transcription. Dissociation of the early transcription factor-promoter complex. J Biol Chem. 1991 Aug 15;266(23):15545–15548. [PubMed] [Google Scholar]
- Broyles S. S., Fesler B. S. Vaccinia virus gene encoding a component of the viral early transcription factor. J Virol. 1990 Apr;64(4):1523–1529. doi: 10.1128/jvi.64.4.1523-1529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Li J., Moss B. Promoter DNA contacts made by the vaccinia virus early transcription factor. J Biol Chem. 1991 Aug 15;266(23):15539–15544. [PubMed] [Google Scholar]
- Broyles S. S., Moss B. DNA-dependent ATPase activity associated with vaccinia virus early transcription factor. J Biol Chem. 1988 Aug 5;263(22):10761–10765. [PubMed] [Google Scholar]
- Broyles S. S., Yuen L., Shuman S., Moss B. Purification of a factor required for transcription of vaccinia virus early genes. J Biol Chem. 1988 Aug 5;263(22):10754–10760. [PubMed] [Google Scholar]
- Carpenter M. S., DeLange A. M. A temperature-sensitive lesion in the small subunit of the vaccinia virus-encoded mRNA capping enzyme causes a defect in viral telomere resolution. J Virol. 1991 Aug;65(8):4042–4050. doi: 10.1128/jvi.65.8.4042-4050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M. S., DeLange A. M. Identification of a temperature-sensitive mutant of vaccinia virus defective in late but not intermediate gene expression. Virology. 1992 May;188(1):233–244. doi: 10.1016/0042-6822(92)90753-c. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cochran M. A., Mackett M., Moss B. Eukaryotic transient expression system dependent on transcription factors and regulatory DNA sequences of vaccinia virus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):19–23. doi: 10.1073/pnas.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Puckett C., Moss B. In vitro mutagenesis of the promoter region for a vaccinia virus gene: evidence for tandem early and late regulatory signals. J Virol. 1985 Apr;54(1):30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Milovanovitch V., Pogo B. G., Weintraub S. B., Huima T., Wilton S., McFadden G. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology. 1978 Feb;84(2):403–428. doi: 10.1016/0042-6822(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989 Dec 20;210(4):749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- DeLange A. M. Identification of temperature-sensitive mutants of vaccinia virus that are defective in conversion of concatemeric replicative intermediates to the mature linear DNA genome. J Virol. 1989 Jun;63(6):2437–2444. doi: 10.1128/jvi.63.6.2437-2444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. A., Smith G. L. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J Virol. 1992 Mar;66(3):1610–1621. doi: 10.1128/jvi.66.3.1610-1621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Moss B. Cytoplasmic expression system based on constitutive synthesis of bacteriophage T7 RNA polymerase in mammalian cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6743–6747. doi: 10.1073/pnas.87.17.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J. Phenotypic characterization of temperature-sensitive mutants of vaccinia virus with mutations in a 135,000-Mr subunit of the virion-associated DNA-dependent RNA polymerase. J Virol. 1987 Jun;61(6):1842–1850. doi: 10.1128/jvi.61.6.1842-1850.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Transient dominant selection of recombinant vaccinia viruses. J Virol. 1990 Jun;64(6):3108–3111. doi: 10.1128/jvi.64.6.3108-3111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Fernandez M. P., Moss B. Transfer of the inducible lac repressor/operator system from Escherichia coli to a vaccinia virus expression vector. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2549–2553. doi: 10.1073/pnas.86.8.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon P. D., Moss B. Early transcription factor subunits are encoded by vaccinia virus late genes. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4401–4405. doi: 10.1073/pnas.87.11.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschmann P., Vos J. C., Stunnenberg H. G. Mutational analysis of a vaccinia virus intermediate promoter in vivo and in vitro. J Virol. 1990 Dec;64(12):6063–6069. doi: 10.1128/jvi.64.12.6063-6069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooda-Dhingra U., Patel D. D., Pickup D. J., Condit R. C. Fine structure mapping and phenotypic analysis of five temperature-sensitive mutations in the second largest subunit of vaccinia virus DNA-dependent RNA polymerase. Virology. 1990 Jan;174(1):60–69. doi: 10.1016/0042-6822(90)90054-u. [DOI] [PubMed] [Google Scholar]
- Hooda-Dhingra U., Thompson C. L., Condit R. C. Detailed phenotypic characterization of five temperature-sensitive mutants in the 22- and 147-kilodalton subunits of vaccinia virus DNA-dependent RNA polymerase. J Virol. 1989 Feb;63(2):714–729. doi: 10.1128/jvi.63.2.714-729.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Jones E. V., Moss B. Transcriptional mapping of the vaccinia virus DNA polymerase gene. J Virol. 1985 Jan;53(1):312–315. doi: 10.1128/jvi.53.1.312-315.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. Y., Bauer W. R. Biosynthesis and phosphorylation of vaccinia virus structural protein VP11. Virology. 1987 Aug;159(2):399–407. doi: 10.1016/0042-6822(87)90479-x. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck J. G., Baldick C. J., Jr, Moss B. Role of DNA replication in vaccinia virus gene expression: a naked template is required for transcription of three late trans-activator genes. Cell. 1990 Jun 1;61(5):801–809. doi: 10.1016/0092-8674(90)90190-p. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M., Moss B. Resolution of vaccinia virus DNA concatemer junctions requires late-gene expression. J Virol. 1989 Apr;63(4):1595–1603. doi: 10.1128/jvi.63.4.1595-1603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Ahn B. Y., Amegadzie B., Gershon P. D., Keck J. G. Cytoplasmic transcription system encoded by vaccinia virus. J Biol Chem. 1991 Jan 25;266(3):1355–1358. [PubMed] [Google Scholar]
- Moss B., Elroy-Stein O., Mizukami T., Alexander W. A., Fuerst T. R. Product review. New mammalian expression vectors. Nature. 1990 Nov 1;348(6296):91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- Moss B., Salzman N. P. Sequential protein synthesis following vaccinia virus infection. J Virol. 1968 Oct;2(10):1016–1027. doi: 10.1128/jvi.2.10.1016-1027.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington T. H. Vaccinia virus polypeptide synthesis: sequential appearance and stability of pre- and post-replicative polypeptides. J Gen Virol. 1974 Dec;25(3):433–444. doi: 10.1099/0022-1317-25-3-433. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. F., Smith G. L. Inducible gene expression from vaccinia virus vectors. Virology. 1990 Jul;177(1):239–250. doi: 10.1016/0042-6822(90)90477-9. [DOI] [PubMed] [Google Scholar]
- Sadler J. R., Sasmor H., Betz J. L. A perfectly symmetric lac operator binds the lac repressor very tightly. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6785–6789. doi: 10.1073/pnas.80.22.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto J., Celenza L. M., Condit R. C., Niles E. G. Genetic map of the vaccinia virus HindIII D Fragment. Virology. 1987 Sep;160(1):110–119. doi: 10.1016/0042-6822(87)90051-1. [DOI] [PubMed] [Google Scholar]
- Thompson C. L., Hooda-Dhingra U., Condit R. C. Fine structure mapping of five temperature-sensitive mutants in the 22- and 147-kilodalton subunits of vaccinia virus DNA-dependent RNA polymerase. J Virol. 1989 Feb;63(2):705–713. doi: 10.1128/jvi.63.2.705-713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktman P., Sridhar P., Condit R. C., Roberts B. E. Transcriptional mapping of the DNA polymerase gene of vaccinia virus. J Virol. 1984 Jan;49(1):125–131. doi: 10.1128/jvi.49.1.125-131.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. C., Sasker M., Stunnenberg H. G. Promoter melting by a stage-specific vaccinia virus transcription factor is independent of the presence of RNA polymerase. Cell. 1991 Apr 5;65(1):105–113. doi: 10.1016/0092-8674(91)90412-r. [DOI] [PubMed] [Google Scholar]
- Vos J. C., Sasker M., Stunnenberg H. G. Vaccinia virus capping enzyme is a transcription initiation factor. EMBO J. 1991 Sep;10(9):2553–2558. doi: 10.1002/j.1460-2075.1991.tb07795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. C., Stunnenberg H. G. Derepression of a novel class of vaccinia virus genes upon DNA replication. EMBO J. 1988 Nov;7(11):3487–3492. doi: 10.1002/j.1460-2075.1988.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylated nucleotides block 5'-terminus of vaccinia virus messenger RNA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):318–322. doi: 10.1073/pnas.72.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Hänggi M., Hiller G. Mapping of a gene coding for a major late structural polypeptide on the vaccinia virus genome. J Virol. 1984 Feb;49(2):371–378. doi: 10.1128/jvi.49.2.371-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. F., Keck J. G., Tsai M. M., Moss B. A transcription factor for expression of vaccinia virus late genes is encoded by an intermediate gene. J Virol. 1991 Jul;65(7):3715–3720. doi: 10.1128/jvi.65.7.3715-3720.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. F., Moss B. Identification of factors specific for transcription of the late class of vaccinia virus genes. J Virol. 1989 Oct;63(10):4224–4233. doi: 10.1128/jvi.63.10.4224-4233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L., Davison A. J., Moss B. Early promoter-binding factor from vaccinia virions. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6069–6073. doi: 10.1073/pnas.84.17.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L., Moss B. Multiple 3' ends of mRNA encoding vaccinia virus growth factor occur within a series of repeated sequences downstream of T clusters. J Virol. 1986 Oct;60(1):320–323. doi: 10.1128/jvi.60.1.320-323.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Moss B. Inducer-dependent conditional-lethal mutant animal viruses. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1511–1515. doi: 10.1073/pnas.88.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Moss B. Vaccinia virus morphogenesis is interrupted when expression of the gene encoding an 11-kilodalton phosphorylated protein is prevented by the Escherichia coli lac repressor. J Virol. 1991 Nov;65(11):6101–6110. doi: 10.1128/jvi.65.11.6101-6110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Moss B. Immature viral envelope formation is interrupted at the same stage by lac operator-mediated repression of the vaccinia virus D13L gene and by the drug rifampicin. Virology. 1992 Apr;187(2):643–653. doi: 10.1016/0042-6822(92)90467-4. [DOI] [PubMed] [Google Scholar]