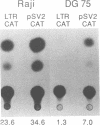
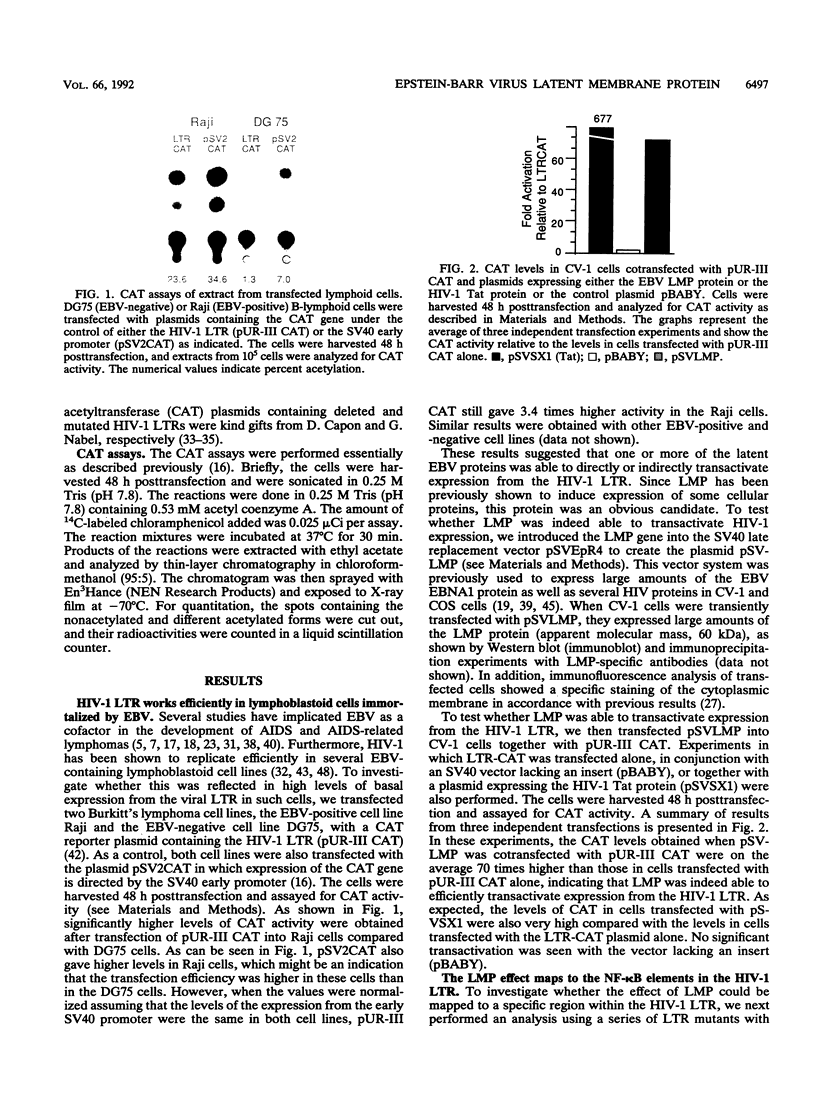
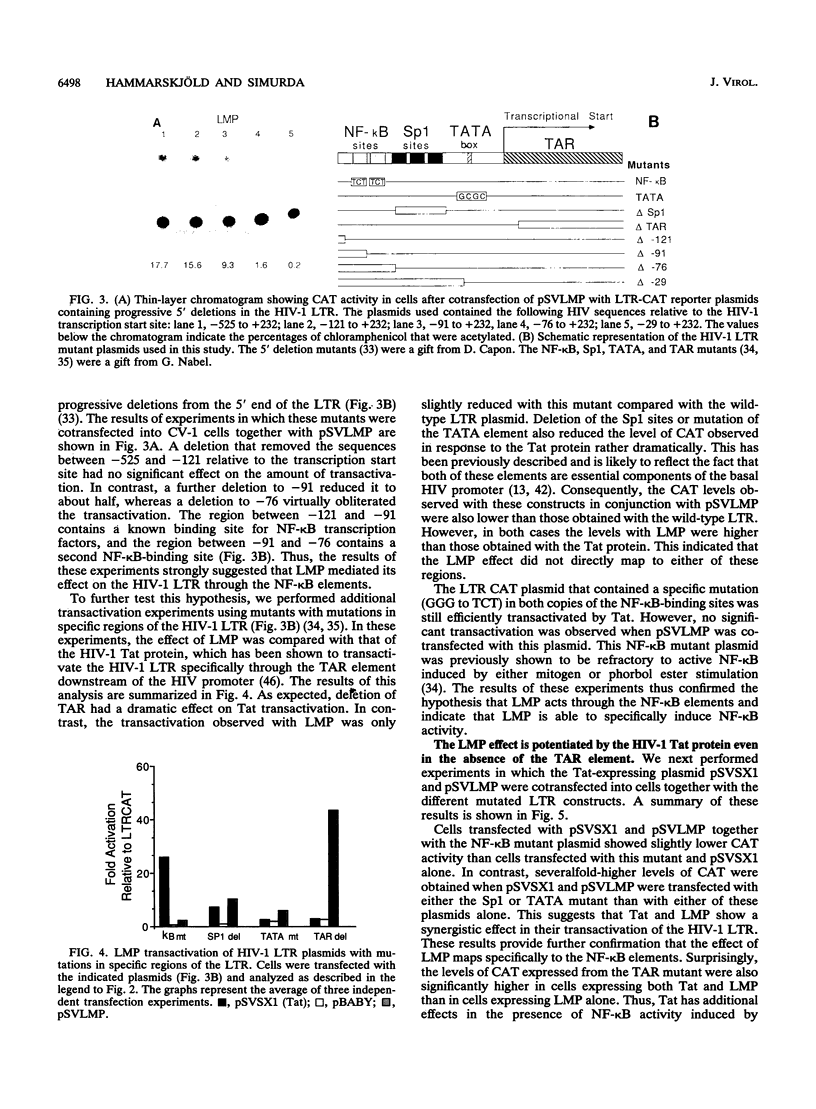
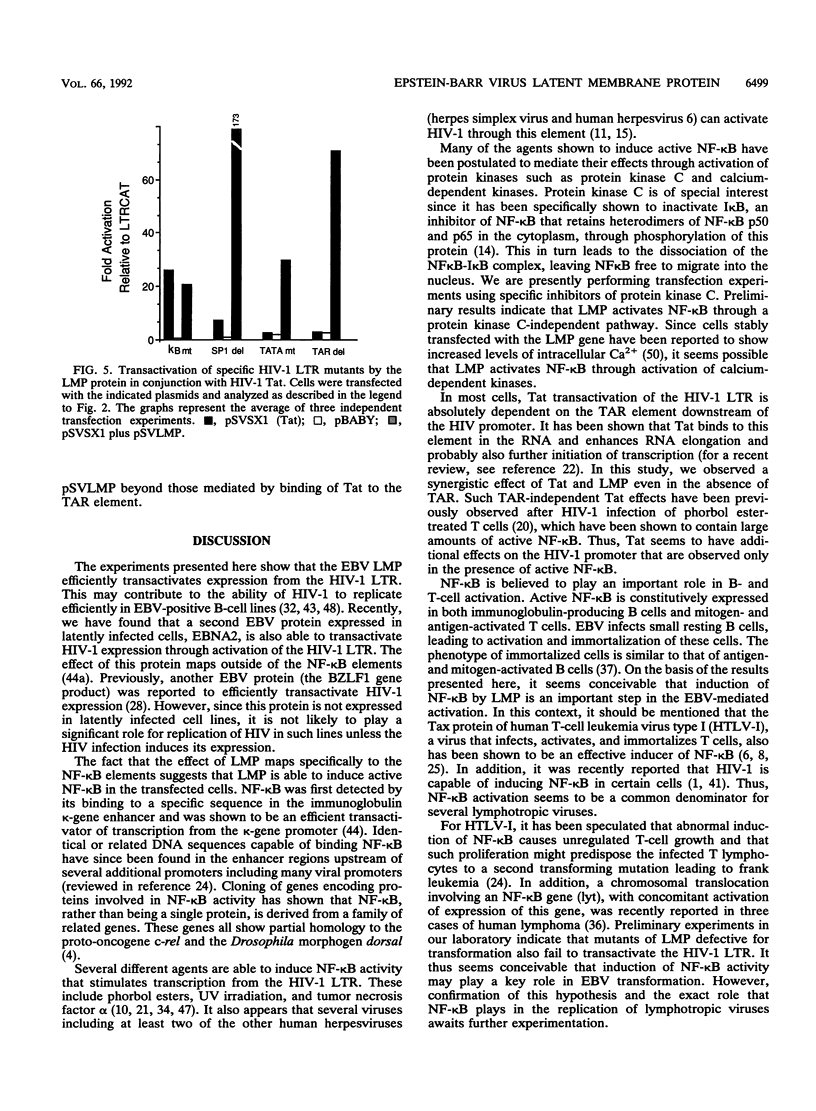
Abstract
The Epstein-Barr virus latent membrane protein (LMP) is an integral membrane protein that is expressed in cells latently infected with the virus. LMP is believed to play an important role in Epstein-Barr virus transformation and has been shown to induce expression of several cellular proteins. We performed a series of experiments that demonstrated that LMP is an efficient transactivator of expression from the human immunodeficiency virus type 1 long terminal repeat (HIV-1 LTR). Mutation or deletion of the NF-kappa B elements in the LTR abolished the transactivation, indicating that the LMP effect on HIV expression was due to induction of NF-kappa B activity. Experiments in which the HIV-1 Tat protein was coexpressed in cells together with LMP showed that Tat was able to potentiate the transactivation. Surprisingly, a synergistic effect of the two proteins was observed even in the absence of the recognized target region for Tat (TAR) in the HIV-1 LTR.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachelerie F., Alcami J., Arenzana-Seisdedos F., Virelizier J. L. HIV enhancer activity perpetuated by NF-kappa B induction on infection of monocytes. Nature. 1991 Apr 25;350(6320):709–712. doi: 10.1038/350709a0. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Hammerschmidt W., Sugden B. Characterization of the BNLF-1 oncogene of Epstein-Barr virus. Curr Top Microbiol Immunol. 1989;144:233–239. doi: 10.1007/978-3-642-74578-2_29. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. The multiple membrane-spanning segments of the BNLF-1 oncogene from Epstein-Barr virus are required for transformation. Oncogene. 1989 Jan;4(1):67–74. [PubMed] [Google Scholar]
- Ballard D. W., Walker W. H., Doerre S., Sista P., Molitor J. A., Dixon E. P., Peffer N. J., Hannink M., Greene W. C. The v-rel oncogene encodes a kappa B enhancer binding protein that inhibits NF-kappa B function. Cell. 1990 Nov 16;63(4):803–814. doi: 10.1016/0092-8674(90)90146-6. [DOI] [PubMed] [Google Scholar]
- Birx D. L., Redfield R. R., Tosato G. Defective regulation of Epstein-Barr virus infection in patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related disorders. N Engl J Med. 1986 Apr 3;314(14):874–879. doi: 10.1056/NEJM198604033141403. [DOI] [PubMed] [Google Scholar]
- Boyle M. J., Sewell W. A., Sculley T. B., Apolloni A., Turner J. J., Swanson C. E., Penny R., Cooper D. A. Subtypes of Epstein-Barr virus in human immunodeficiency virus-associated non-Hodgkin lymphoma. Blood. 1991 Dec 1;78(11):3004–3011. [PubMed] [Google Scholar]
- Böhnlein E., Siekevitz M., Ballard D. W., Lowenthal J. W., Rimsky L., Bogérd H., Hoffman J., Wano Y., Franza B. R., Greene W. C. Stimulation of the human immunodeficiency virus type 1 enhancer by the human T-cell leukemia virus type I tax gene product involves the action of inducible cellular proteins. J Virol. 1989 Apr;63(4):1578–1586. doi: 10.1128/jvi.63.4.1578-1586.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. L., Halden N. F., Lenardo M. J., Leonard W. J. Functionally distinct NF-kappa B binding sites in the immunoglobulin kappa and IL-2 receptor alpha chain genes. Science. 1989 Apr 28;244(4903):466–469. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- Dawson C. W., Rickinson A. B., Young L. S. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature. 1990 Apr 19;344(6268):777–780. doi: 10.1038/344777a0. [DOI] [PubMed] [Google Scholar]
- Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B., Lusso P., Schachter F., Josephs S. F., Rappaport J., Negro F., Gallo R. C., Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989 Oct;8(10):3019–3027. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. A., Wu F. K., Mitsuyasu R., Gaynor R. B. Interactions of cellular proteins involved in the transcriptional regulation of the human immunodeficiency virus. EMBO J. 1987 Dec 1;6(12):3761–3770. doi: 10.1002/j.1460-2075.1987.tb02711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Gimble J. M., Duh E., Ostrove J. M., Gendelman H. E., Max E. E., Rabson A. B. Activation of the human immunodeficiency virus long terminal repeat by herpes simplex virus type 1 is associated with induction of a nuclear factor that binds to the NF-kappa B/core enhancer sequence. J Virol. 1988 Nov;62(11):4104–4112. doi: 10.1128/jvi.62.11.4104-4112.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner J., del Rio C., Carr D., Hendrix L. E., Eley J. W., Unger E. R. Non-Hodgkin's lymphomas in patients with human immunodeficiency virus infection. Presence of Epstein-Barr virus by in situ hybridization, clinical presentation, and follow-up. Cancer. 1991 Dec 1;68(11):2460–2465. doi: 10.1002/1097-0142(19911201)68:11<2460::aid-cncr2820681123>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Hamilton-Dutoit S. J., Pallesen G., Franzmann M. B., Karkov J., Black F., Skinhøj P., Pedersen C. AIDS-related lymphoma. Histopathology, immunophenotype, and association with Epstein-Barr virus as demonstrated by in situ nucleic acid hybridization. Am J Pathol. 1991 Jan;138(1):149–163. [PMC free article] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Wang S. C., Klein G. High-level expression of the Epstein-Barr virus EBNA1 protein in CV1 cells and human lymphoid cells using a SV40 late replacement vector. Gene. 1986;43(1-2):41–50. doi: 10.1016/0378-1119(86)90006-5. [DOI] [PubMed] [Google Scholar]
- Harrich D., Garcia J., Mitsuyasu R., Gaynor R. TAR independent activation of the human immunodeficiency virus in phorbol ester stimulated T lymphocytes. EMBO J. 1990 Dec;9(13):4417–4423. doi: 10.1002/j.1460-2075.1990.tb07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann H. P., Brockhaus M., Baeuerle P. A., Remy R., Kolbeck R., van Loon A. P. Expression of the types A and B tumor necrosis factor (TNF) receptors is independently regulated, and both receptors mediate activation of the transcription factor NF-kappa B. TNF alpha is not needed for induction of a biological effect via TNF receptors. J Biol Chem. 1990 Dec 25;265(36):22409–22417. [PubMed] [Google Scholar]
- Jeang K. T., Chang Y., Berkhout B., Hammarskjöld M. L., Rekosh D. Regulation of HIV expression: mechanisms of action of Tat and Rev. AIDS. 1991;5 (Suppl 2):S3–14. [PubMed] [Google Scholar]
- Laurence J., Astrin S. M. Human immunodeficiency virus induction of malignant transformation in human B lymphocytes. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7635–7639. doi: 10.1073/pnas.88.17.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Leung K., Nabel G. J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988 Jun 23;333(6175):776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- Liebowitz D., Kopan R., Fuchs E., Sample J., Kieff E. An Epstein-Barr virus transforming protein associates with vimentin in lymphocytes. Mol Cell Biol. 1987 Jul;7(7):2299–2308. doi: 10.1128/mcb.7.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz D., Wang D., Kieff E. Orientation and patching of the latent infection membrane protein encoded by Epstein-Barr virus. J Virol. 1986 Apr;58(1):233–237. doi: 10.1128/jvi.58.1.233-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon R., Borkowski J., Albin R., Pepitoni S., Schwartz J., Kieff E. The Epstein-Barr virus BZLF1 gene product activates the human immunodeficiency virus type 1 5' long terminal repeat. J Virol. 1990 Dec;64(12):6282–6285. doi: 10.1128/jvi.64.12.6282-6285.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Sugden B. Transformation by the oncogenic latent membrane protein correlates with its rapid turnover, membrane localization, and cytoskeletal association. J Virol. 1991 Jun;65(6):3246–3258. doi: 10.1128/jvi.65.6.3246-3258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker T. C., Shiramizu B., Kaplan L., Herndier B., Sanchez H., Grimaldi J. C., Baumgartner J., Rachlin J., Feigal E., Rosenblum M. Evidence for molecular subtypes of HIV-associated lymphoma: division into peripheral monoclonal, polyclonal and central nervous system lymphoma. AIDS. 1991 Jun;5(6):669–674. [PubMed] [Google Scholar]
- Monroe J. E., Calender A., Mulder C. Epstein-Barr virus-positive and -negative B-cell lines can be infected with human immunodeficiency virus types 1 and 2. J Virol. 1988 Sep;62(9):3497–3500. doi: 10.1128/jvi.62.9.3497-3500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Nabel G. J., Rice S. A., Knipe D. M., Baltimore D. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science. 1988 Mar 11;239(4845):1299–1302. doi: 10.1126/science.2830675. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Neri A., Chang C. C., Lombardi L., Salina M., Corradini P., Maiolo A. T., Chaganti R. S., Dalla-Favera R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell. 1991 Dec 20;67(6):1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Klein G. Phenotypic and cytogenetic characteristics of human B-lymphoid cell lines and their relevance for the etiology of Burkitt's lymphoma. Adv Cancer Res. 1982;37:319–380. doi: 10.1016/s0065-230x(08)60886-6. [DOI] [PubMed] [Google Scholar]
- Pedersen C., Gerstoft J., Lundgren J. D., Skinhøj P., Bøttzauw J., Geisler C., Hamilton-Dutoit S. J., Thorsen S., Lisse I., Ralfkiaer E. HIV-associated lymphoma: histopathology and association with Epstein-Barr virus genome related to clinical, immunological and prognostic features. Eur J Cancer. 1991;27(11):1416–1423. doi: 10.1016/0277-5379(91)90023-7. [DOI] [PubMed] [Google Scholar]
- Rekosh D., Nygren A., Flodby P., Hammarskjöld M. L., Wigzell H. Coexpression of human immunodeficiency virus envelope proteins and tat from a single simian virus 40 late replacement vector. Proc Natl Acad Sci U S A. 1988 Jan;85(2):334–338. doi: 10.1073/pnas.85.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Kingsley L. A., Lyter D. W., Rabin B. S., Atchison R. W., Bodner A. J., Weiss S. H., Saxinger W. C. Association of HTLV-III with Epstein-Barr virus infection and abnormalities of T lymphocytes in homosexual men. J Infect Dis. 1986 Oct;154(4):556–561. doi: 10.1093/infdis/154.4.556. [DOI] [PubMed] [Google Scholar]
- Rivière Y., Blank V., Kourilsky P., Israël A. Processing of the precursor of NF-kappa B by the HIV-1 protease during acute infection. Nature. 1991 Apr 18;350(6319):625–626. doi: 10.1038/350625a0. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Hunter E. A., Gonda M. A., Sturzenegger S., Markham P. D., Gallo R. C. HTLV-III infection of EBV-genome-positive B-lymphoid cells with or without detectable T4 antigens. Int J Cancer. 1987 Feb 15;39(2):198–202. doi: 10.1002/ijc.2910390213. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Cho M. I., Hammarskjöld M. L., Rekosh D. Human immunodeficiency virus type 1 Pr55gag and Pr160gag-pol expressed from a simian virus 40 late replacement vector are efficiently processed and assembled into viruslike particles. J Virol. 1990 Jun;64(6):2743–2750. doi: 10.1128/jvi.64.6.2743-2750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Stein B., Krämer M., Rahmsdorf H. J., Ponta H., Herrlich P. UV-induced transcription from the human immunodeficiency virus type 1 (HIV-1) long terminal repeat and UV-induced secretion of an extracellular factor that induces HIV-1 transcription in nonirradiated cells. J Virol. 1989 Nov;63(11):4540–4544. doi: 10.1128/jvi.63.11.4540-4544.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V., Britton S., Ehrnst A., Lenkei R., Strannegård O. Persistent productive HIV infection in EBV-transformed B lymphocytes. J Med Virol. 1989 Jan;27(1):19–24. doi: 10.1002/jmv.1890270105. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. B., Weinberg W., Johnson R., Yuspa S., Levine A. J. Expression of the BNLF-1 oncogene of Epstein-Barr virus in the skin of transgenic mice induces hyperplasia and aberrant expression of keratin 6. Cell. 1990 Jun 29;61(7):1315–1327. doi: 10.1016/0092-8674(90)90695-b. [DOI] [PubMed] [Google Scholar]