Abstract
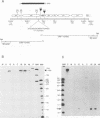
We identified a gene of Autographa californica nuclear polyhedrosis virus (AcMNPV) that encodes a small cysteine-rich polypeptide which has size and sequence similarity to omega-conotoxins, a class of calcium ion (Ca2+) channel inhibitors, found in the venom of cone snails. Transcriptional analysis indicated that the 159-bp open reading frame, which we named ctl, and a downstream 984-bp open reading frame are transcribed as a single 1.3-kb bicistronic late RNA. The mature ctl gene product was identified as a small secreted protein by high-pressure liquid chromatography fractionation of extracellular fluid. Viruses with a site-specific deletion in ctl appeared normal with regard to the kinetics and virulence of infection, both in vitro and in vivo. Although we studied the behavior of wild-type and mutant virus-infected insects in some detail, a biological role for ctl in AcMNPV infection remains to be established.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. E., Bindokas V. P., Hasegawa L., Venema V. J. Omega-agatoxins: novel calcium channel antagonists of two subtypes from funnel web spider (Agelenopsis aperta) venom. J Biol Chem. 1990 Jan 15;265(2):861–867. [PubMed] [Google Scholar]
- Blissard G. W., Rohrmann G. F. Baculovirus diversity and molecular biology. Annu Rev Entomol. 1990;35:127–155. doi: 10.1146/annurev.en.35.010190.001015. [DOI] [PubMed] [Google Scholar]
- Blumenthal K. M., Kem W. R. Structure and action of heteronemertine polypeptide toxins: disulfide bonds of Cerebratulus lacteus toxin B-IV. J Biol Chem. 1977 May 25;252(10):3328–3331. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bontems F., Roumestand C., Gilquin B., Ménez A., Toma F. Refined structure of charybdotoxin: common motifs in scorpion toxins and insect defensins. Science. 1991 Dec 6;254(5037):1521–1523. doi: 10.1126/science.1720574. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cruz L. J., Olivera B. M. Calcium channel antagonists. Omega-conotoxin defines a new high affinity site. J Biol Chem. 1986 May 15;261(14):6230–6233. [PubMed] [Google Scholar]
- Darbon H., Weber C., Braun W. Two-dimensional 1H nuclear magnetic resonance study of AaH IT, an anti-insect toxin from the scorpion Androctonus australis Hector. Sequential resonance assignments and folding of the polypeptide chain. Biochemistry. 1991 Feb 19;30(7):1836–1845. doi: 10.1021/bi00221a016. [DOI] [PubMed] [Google Scholar]
- Darbon H., Zlotkin E., Kopeyan C., van Rietschoten J., Rochat H. Covalent structure of the insect toxin of the North African scorpion Androctonus australis Hector. Int J Pept Protein Res. 1982 Oct;20(4):320–330. doi: 10.1111/j.1399-3011.1982.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Hardin S. E., Weaver R. F. Overlapping divergent transcripts mapping to the HindIII F region of the Autographa californica nuclear polyhedrosis virus. J Gen Virol. 1990 Jan;71(Pt 1):225–229. doi: 10.1099/0022-1317-71-1-225. [DOI] [PubMed] [Google Scholar]
- Harlow P., Watkins E., Thornton R. D., Nemer M. Structure of an ectodermally expressed sea urchin metallothionein gene and characterization of its metal-responsive region. Mol Cell Biol. 1989 Dec;9(12):5445–5455. doi: 10.1128/mcb.9.12.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard D. R., Olivera B. M., Woodward S., Corpuz G. P., Gray W. R., Ramilo C. A., Cruz L. J. A molluscivorous Conus toxin: conserved frameworks in conotoxins. Biochemistry. 1989 Jan 10;28(1):358–361. doi: 10.1021/bi00427a049. [DOI] [PubMed] [Google Scholar]
- Kovacs G. R., Guarino L. A., Summers M. D. Novel regulatory properties of the IE1 and IE0 transactivators encoded by the baculovirus Autographa californica multicapsid nuclear polyhedrosis virus. J Virol. 1991 Oct;65(10):5281–5288. doi: 10.1128/jvi.65.10.5281-5288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Schäfer U., Schäfer M. Cis-acting regions sufficient for spermatocyte-specific transcriptional and spermatid-specific translational control of the Drosophila melanogaster gene mst(3)gl-9. EMBO J. 1988 Feb;7(2):447–454. doi: 10.1002/j.1460-2075.1988.tb02832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITCHFIELD J. T., Jr A method for rapid graphic solution of time-per cent effect curves. J Pharmacol Exp Ther. 1949 Dec;97(4):399-408, 3 tab. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee H. H., Miller L. K. Isolation of genotypic variants of Autographa californica nuclear polyhedrosis virus. J Virol. 1978 Sep;27(3):754–767. doi: 10.1128/jvi.27.3.754-767.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G. S., Maillard M., Kiraly M. Ion channels involved in the presynaptic hyperexcitability induced by herpes virus suis in rat superior cervical ganglion. Neuroscience. 1991;41(2-3):797–807. doi: 10.1016/0306-4522(91)90370-4. [DOI] [PubMed] [Google Scholar]
- Maehlen J., Wallén P., Löve A., Norrby E., Kristensson K. Paramyxovirus infections alter certain functional properties in cultured sensory neurons. Brain Res. 1991 Feb 1;540(1-2):123–130. doi: 10.1016/0006-8993(91)90498-k. [DOI] [PubMed] [Google Scholar]
- Martin M. F., Garcia y Perez L. G., el Ayeb M., Kopeyan C., Bechis G., Jover E., Rochat H. Purification and chemical and biological characterizations of seven toxins from the Mexican scorpion, Centruroides suffusus suffusus. J Biol Chem. 1987 Apr 5;262(10):4452–4459. [PubMed] [Google Scholar]
- Nojiri H., Ishida I., Miyashita E., Sato M., Urano A., Deguchi T. Cloning and sequence analysis of cDNAs for neurohypophysial hormones vasotocin and mesotocin for the hypothalamus of toad, Bufo japonicus. Proc Natl Acad Sci U S A. 1987 May;84(9):3043–3046. doi: 10.1073/pnas.84.9.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly D. R., Miller L. K. A baculovirus blocks insect molting by producing ecdysteroid UDP-glucosyl transferase. Science. 1989 Sep 8;245(4922):1110–1112. doi: 10.1126/science.2505387. [DOI] [PubMed] [Google Scholar]
- O'Reilly D. R., Passarelli A. L., Goldman I. F., Miller L. K. Characterization of the DA26 gene in a hypervariable region of the Autographa californica nuclear polyhedrosis virus genome. J Gen Virol. 1990 May;71(Pt 5):1029–1037. doi: 10.1099/0022-1317-71-5-1029. [DOI] [PubMed] [Google Scholar]
- Oellig C., Happ B., Müller T., Doerfler W. Overlapping sets of viral RNAs reflect the array of polypeptides in the EcoRI J and N fragments (map positions 81.2 to 85.0) of the Autographa californica nuclear polyhedrosis virus genome. J Virol. 1987 Oct;61(10):3048–3057. doi: 10.1128/jvi.61.10.3048-3057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Cruz L. J., de Santos V., LeCheminant G. W., Griffin D., Zeikus R., McIntosh J. M., Galyean R., Varga J., Gray W. R. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry. 1987 Apr 21;26(8):2086–2090. doi: 10.1021/bi00382a004. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Gray W. R., Zeikus R., McIntosh J. M., Varga J., Rivier J., de Santos V., Cruz L. J. Peptide neurotoxins from fish-hunting cone snails. Science. 1985 Dec 20;230(4732):1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., McIntosh J. M., Cruz L. J., Luque F. A., Gray W. R. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984 Oct 23;23(22):5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Rivier J., Clark C., Ramilo C. A., Corpuz G. P., Abogadie F. C., Mena E. E., Woodward S. R., Hillyard D. R., Cruz L. J. Diversity of Conus neuropeptides. Science. 1990 Jul 20;249(4966):257–263. doi: 10.1126/science.2165278. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Rivier J., Scott J. K., Hillyard D. R., Cruz L. J. Conotoxins. J Biol Chem. 1991 Nov 25;266(33):22067–22070. [PubMed] [Google Scholar]
- Ooi B. G., Rankin C., Miller L. K. Downstream sequences augment transcription from the essential initiation site of a baculovirus polyhedrin gene. J Mol Biol. 1989 Dec 20;210(4):721–736. doi: 10.1016/0022-2836(89)90105-8. [DOI] [PubMed] [Google Scholar]
- Parsons T. J., Bradshaw H. D., Jr, Gordon M. P. Systemic accumulation of specific mRNAs in response to wounding in poplar trees. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7895–7899. doi: 10.1073/pnas.86.20.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L. H., Holsinger L. J., Lamb R. A. Influenza virus M2 protein has ion channel activity. Cell. 1992 May 1;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- Possee R. D., Howard S. C. Analysis of the polyhedrin gene promoter of the Autographa californica nuclear polyhedrosis virus. Nucleic Acids Res. 1987 Dec 23;15(24):10233–10248. doi: 10.1093/nar/15.24.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possee R. D., Sun T. P., Howard S. C., Ayres M. D., Hill-Perkins M., Gearing K. L. Nucleotide sequence of the Autographa californica nuclear polyhedrosis 9.4 kbp EcoRI-I and -R (polyhedrin gene) region. Virology. 1991 Nov;185(1):229–241. doi: 10.1016/0042-6822(91)90770-c. [DOI] [PubMed] [Google Scholar]
- Schweitz H., Bidard J. N., Frelin C., Pauron D., Vijverberg H. P., Mahasneh D. M., Lazdunski M., Vilbois F., Tsugita A. Purification, sequence, and pharmacological properties of sea anemone toxins from Radianthus paumotensis. A new class of sea anemone toxins acting on the sodium channel. Biochemistry. 1985 Jul 2;24(14):3554–3561. doi: 10.1021/bi00335a025. [DOI] [PubMed] [Google Scholar]
- Stauffer E. K., Ziegler R. J. Loss of functional voltage-gated sodium channels in persistent mumps virus-infected PC12 cells. J Gen Virol. 1989 Mar;70(Pt 3):749–754. doi: 10.1099/0022-1317-70-3-749. [DOI] [PubMed] [Google Scholar]
- Thiem S. M., Miller L. K. Identification, sequence, and transcriptional mapping of the major capsid protein gene of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol. 1989 May;63(5):2008–2018. doi: 10.1128/jvi.63.5.2008-2018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilakaratne N., Hardin S. E., Weaver R. F. Nucleotide sequence and transcript mapping of the HindIII F region of the Autographa californica nuclear polyhedrosis virus genome. J Gen Virol. 1991 Feb;72(Pt 2):285–291. doi: 10.1099/0022-1317-72-2-285. [DOI] [PubMed] [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 1977 Apr;13(4):213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- Wang X. Z., Ooi B. G., Miller L. K. Baculovirus vectors for multiple gene expression and for occluded virus production. Gene. 1991 Apr;100:131–137. doi: 10.1016/0378-1119(91)90358-i. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward S. R., Cruz L. J., Olivera B. M., Hillyard D. R. Constant and hypervariable regions in conotoxin propeptides. EMBO J. 1990 Apr;9(4):1015–1020. doi: 10.1002/j.1460-2075.1990.tb08204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]