Abstract
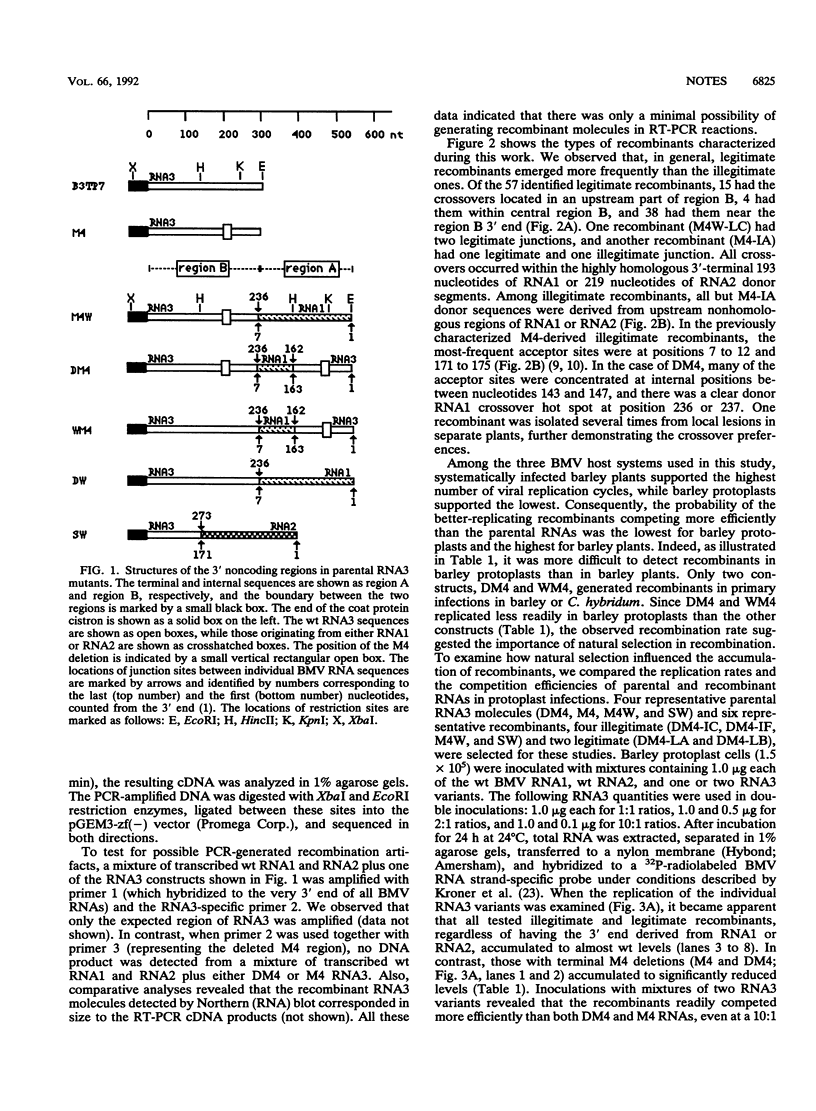
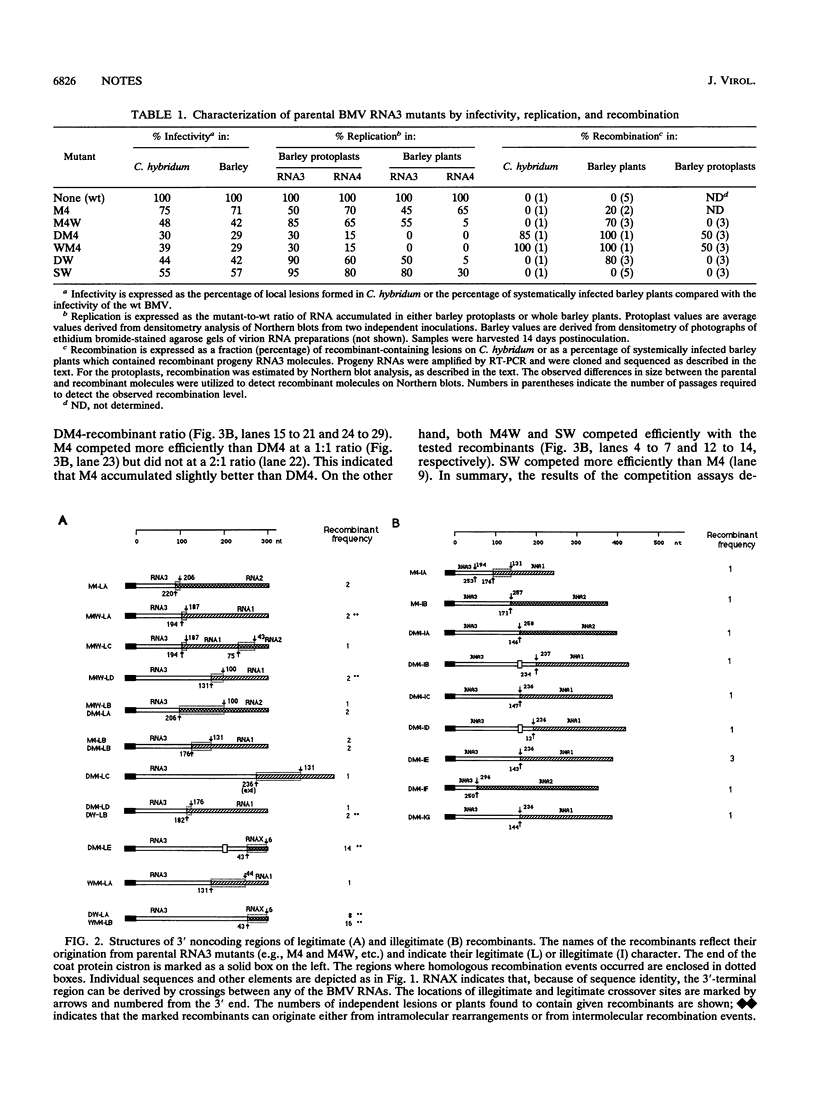
In order to facilitate the isolation of recombinants in brome mosaic virus, a series of duplication mutants with alterations in the RNA3 3' noncoding region has been engineered. The distribution of crossovers, which was observed to be dependent on the parental RNA3 sequence, supported the role of RNA structure in recombination. However, a negative correlation between replication of the parental RNA3 constructs and the accumulation of recombinant progeny confirmed the role of selection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Near identity of 3- RNA secondary structure in bromoviruses and cucumber mosaic virus. Cell. 1981 Jan;23(1):183–189. doi: 10.1016/0092-8674(81)90283-x. [DOI] [PubMed] [Google Scholar]
- Allison R., Thompson C., Ahlquist P. Regeneration of a functional RNA virus genome by recombination between deletion mutants and requirement for cowpea chlorotic mottle virus 3a and coat genes for systemic infection. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1820–1824. doi: 10.1073/pnas.87.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenent G. C., Linthorst H. J., van Belkum A. F., Cornelissen B. J., Bol J. F. RNA 2 of tobacco rattle virus strain TCM encodes an unexpected gene. Nucleic Acids Res. 1986 Jun 11;14(11):4673–4682. doi: 10.1093/nar/14.11.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner L. R., Keck J. G., Lai M. M. A clustering of RNA recombination sites adjacent to a hypervariable region of the peplomer gene of murine coronavirus. Virology. 1990 Apr;175(2):548–555. doi: 10.1016/0042-6822(90)90439-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner L. R., Lai M. M. Random nature of coronavirus RNA recombination in the absence of selection pressure. Virology. 1991 Nov;185(1):441–445. doi: 10.1016/0042-6822(91)90795-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzoubaa S., Niesbach-Klösgen U., Jupin I., Guilley H., Richards K., Jonard G. Shortened forms of beet necrotic yellow vein virus RNA-3 and -4: internal deletions and a subgenomic RNA. J Gen Virol. 1991 Feb;72(Pt 2):259–266. doi: 10.1099/0022-1317-72-2-259. [DOI] [PubMed] [Google Scholar]
- Bujarski J. J., Ahlquist P., Hall T. C., Dreher T. W., Kaesberg P. Modulation of replication, aminoacylation and adenylation in vitro and infectivity in vivo of BMV RNAs containing deletions within the multifunctional 3' end. EMBO J. 1986 Aug;5(8):1769–1774. doi: 10.1002/j.1460-2075.1986.tb04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Dreher T. W., Hall T. C. Deletions in the 3'-terminal tRNA-like structure of brome mosaic virus RNA differentially affect aminoacylation and replication in vitro. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5636–5640. doi: 10.1073/pnas.82.17.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Dzianott A. M. Generation and analysis of nonhomologous RNA-RNA recombinants in brome mosaic virus: sequence complementarities at crossover sites. J Virol. 1991 Aug;65(8):4153–4159. doi: 10.1128/jvi.65.8.4153-4159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Kaesberg P. Genetic recombination between RNA components of a multipartite plant virus. 1986 May 29-Jun 4Nature. 321(6069):528–531. doi: 10.1038/321528a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascone P. J., Carpenter C. D., Li X. H., Simon A. E. Recombination between satellite RNAs of turnip crinkle virus. EMBO J. 1990 Jun;9(6):1709–1715. doi: 10.1002/j.1460-2075.1990.tb08294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W. O., Lewandowski D. J., Hilf M. E., Bubrick P., Raffo A. J., Shaw J. J., Grantham G. L., Desjardins P. R. A tobacco mosaic virus-hybrid expresses and loses an added gene. Virology. 1989 Sep;172(1):285–292. doi: 10.1016/0042-6822(89)90130-x. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Bujarski J. J., Hall T. C. Mutant viral RNAs synthesized in vitro show altered aminoacylation and replicase template activities. Nature. 1984 Sep 13;311(5982):171–175. doi: 10.1038/311171a0. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Hall T. C. Mutational analysis of the sequence and structural requirements in brome mosaic virus RNA for minus strand promoter activity. J Mol Biol. 1988 May 5;201(1):31–40. doi: 10.1016/0022-2836(88)90436-6. [DOI] [PubMed] [Google Scholar]
- Goulden M. G., Lomonossoff G. P., Wood K. R., Davies J. W. A model for the generation of tobacco rattle virus (TRV) anomalous isolates: pea early browning virus RNA-2 acquires TRV sequences from both RNA-1 and RNA-2. J Gen Virol. 1991 Jul;72(Pt 7):1751–1754. doi: 10.1099/0022-1317-72-7-1751. [DOI] [PubMed] [Google Scholar]
- Huisman M. J., Cornelissen B. J., Groenendijk C. F., Bol J. F., van Vloten-Doting L. Alfalfa mosaic virus temperature-sensitive mutants. V. The nucleotide sequence of TBTS 7 RNA 3 shows limited nucleotide changes and evidence for heterologous recombination. Virology. 1989 Aug;171(2):409–416. doi: 10.1016/0042-6822(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Kroner P., Ahlquist P., Meshi T. Biological activities of hybrid RNAs generated by 3'-end exchanges between tobacco mosaic and brome mosaic viruses. J Virol. 1991 Jul;65(7):3451–3459. doi: 10.1128/jvi.65.7.3451-3459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda M., French R., Ahlquist P. High efficiency T7 polymerase synthesis of infectious RNA from cloned brome mosaic virus cdna and effects of 5' extensions on transcript infectivity. Virology. 1987 May;158(1):259–262. doi: 10.1016/0042-6822(87)90265-0. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K., Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986 Nov 7;47(3):433–443. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner P., Richards D., Traynor P., Ahlquist P. Defined mutations in a small region of the brome mosaic virus 2 gene cause diverse temperature-sensitive RNA replication phenotypes. J Virol. 1989 Dec;63(12):5302–5309. doi: 10.1128/jvi.63.12.5302-5309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S., Kawamura N., Nomoto A. Genetic variation occurring on the genome of an in vitro insertion mutant of poliovirus type 1. J Virol. 1989 Mar;63(3):1069–1075. doi: 10.1128/jvi.63.3.1069-1075.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Keck J. G., Stohlman S. A., Lai M. M. High-frequency RNA recombination of murine coronaviruses. J Virol. 1986 Mar;57(3):729–737. doi: 10.1128/jvi.57.3.729-737.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo M. A., Jolly C. A. The 5'-terminal sequence of potato leafroll virus RNA: evidence of recombination between virus and host RNA. J Gen Virol. 1991 Oct;72(Pt 10):2591–2595. doi: 10.1099/0022-1317-72-10-2591. [DOI] [PubMed] [Google Scholar]
- Mindich L., Qiao X., Onodera S., Gottlieb P., Strassman J. Heterologous recombination in the double-stranded RNA bacteriophage phi 6. J Virol. 1992 May;66(5):2605–2610. doi: 10.1128/jvi.66.5.2605-2610.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palasingam K., Shaklee P. N. Reversion of Q beta RNA phage mutants by homologous RNA recombination. J Virol. 1992 Apr;66(4):2435–2442. doi: 10.1128/jvi.66.4.2435-2442.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffo A. J., Dawson W. O. Construction of tobacco mosaic virus subgenomic replicons that are replicated and spread systemically in tobacco plants. Virology. 1991 Sep;184(1):277–289. doi: 10.1016/0042-6822(91)90844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. L., Hall T. C. Requirement for a viral trans-acting factor encoded by brome mosaic virus RNA-2 provides strong selection in vivo for functional recombinants. J Virol. 1990 May;64(5):2437–2441. doi: 10.1128/jvi.64.5.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. L., Sullivan B. P., Hall T. C. Use of Chenopodium hybridum facilitates isolation of brome mosaic virus RNA recombinants. J Gen Virol. 1990 Jun;71(Pt 6):1403–1407. doi: 10.1099/0022-1317-71-6-1403. [DOI] [PubMed] [Google Scholar]
- Romanova L. I., Blinov V. M., Tolskaya E. A., Viktorova E. G., Kolesnikova M. S., Guseva E. A., Agol V. I. The primary structure of crossover regions of intertypic poliovirus recombinants: a model of recombination between RNA genomes. Virology. 1986 Nov;155(1):202–213. doi: 10.1016/0042-6822(86)90180-7. [DOI] [PubMed] [Google Scholar]
- Rott M. E., Tremaine J. H., Rochon D. M. Comparison of the 5' and 3' termini of tomato ringspot virus RNA1 and RNA2: evidence for RNA recombination. Virology. 1991 Nov;185(1):468–472. doi: 10.1016/0042-6822(91)90801-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira R., Choi G. H., Hillman B. I., Nuss D. L. The contribution of defective RNAs to the complexity of viral-encoded double-stranded RNA populations present in hypovirulent strains of the chestnut blight fungus Cryphonectria parasitica. EMBO J. 1991 Apr;10(4):741–746. doi: 10.1002/j.1460-2075.1991.tb08005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Strauss E. G. Evolution of RNA viruses. Annu Rev Microbiol. 1988;42:657–683. doi: 10.1146/annurev.mi.42.100188.003301. [DOI] [PubMed] [Google Scholar]
- Tolskaya E. A., Romanova L. I., Blinov V. M., Viktorova E. G., Sinyakov A. N., Kolesnikova M. S., Agol V. I. Studies on the recombination between RNA genomes of poliovirus: the primary structure and nonrandom distribution of crossover regions in the genomes of intertypic poliovirus recombinants. Virology. 1987 Nov;161(1):54–61. doi: 10.1016/0042-6822(87)90170-x. [DOI] [PubMed] [Google Scholar]
- Weiss B. G., Schlesinger S. Recombination between Sindbis virus RNAs. J Virol. 1991 Aug;65(8):4017–4025. doi: 10.1128/jvi.65.8.4017-4025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. X., Cascone P. J., Simon A. E. Recombination between satellite and genomic RNAs of turnip crinkle virus. Virology. 1991 Oct;184(2):791–794. doi: 10.1016/0042-6822(91)90454-j. [DOI] [PubMed] [Google Scholar]
- van der Kuyl A. C., Neeleman L., Bol J. F. Complementation and recombination between alfalfa mosaic virus RNA3 mutants in tobacco plants. Virology. 1991 Aug;183(2):731–738. doi: 10.1016/0042-6822(91)91002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]