Abstract
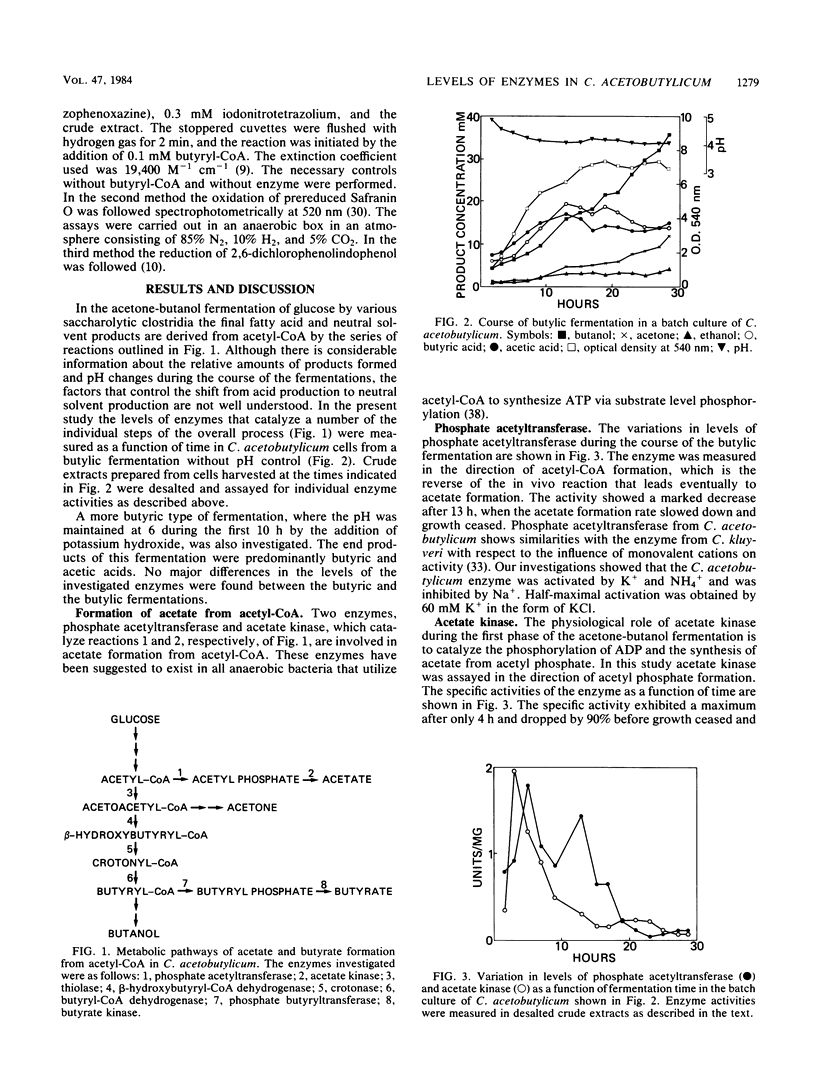
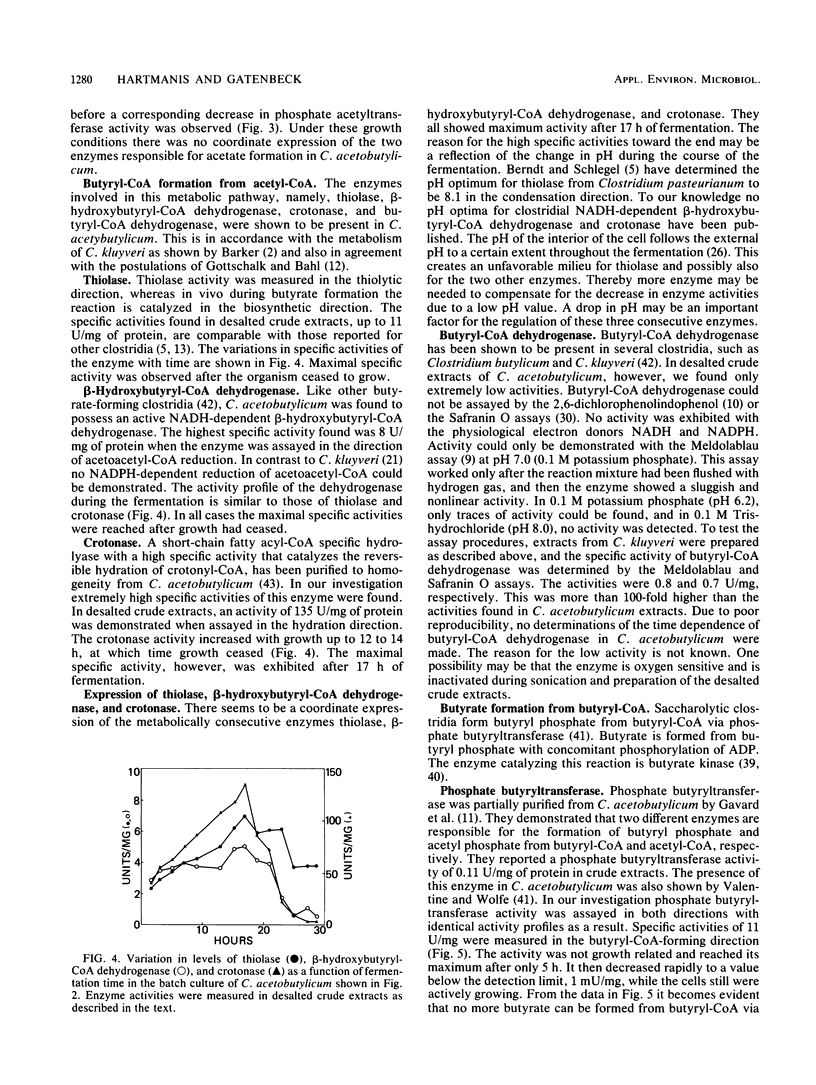
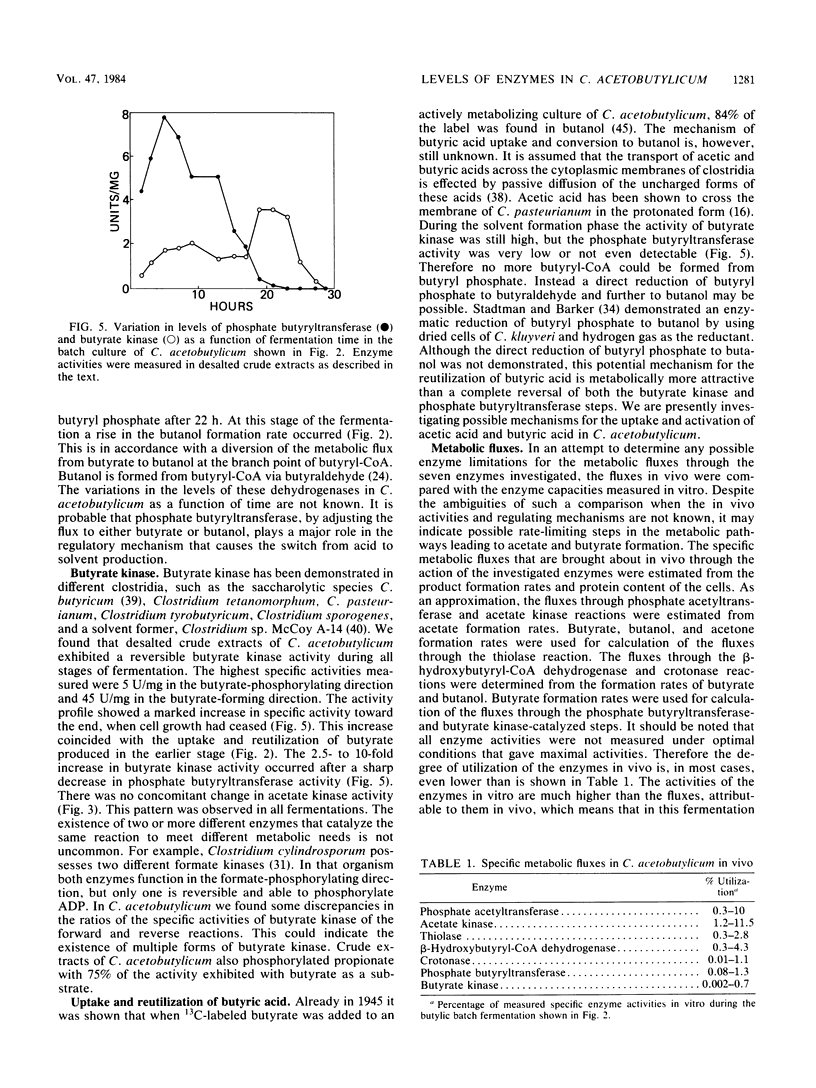
The levels of seven intermediary enzymes involved in acetate and butyrate formation from acetyl coenzyme A in the saccharolytic anaerobe Clostridium acetobutylicum were investigated as a function of time in solvent-producing batch fermentations. Phosphate acetyltransferase and acetate kinase, which are known to form acetate from acetyl coenzyme A, both showed a decrease in specific activity when the organism reached the solvent formation stage. The three consecutive enzymes thiolase, β-hydroxybutyrylcoenzyme A dehydrogenase, and crotonase exhibited a coordinate expression and a maximal activity after growth had ceased. Only low levels of butyryl coenzyme A dehydrogenase activity were found. Phosphate butyryltransferase activity rapidly decreased after 20 h from 5 to 11 U/mg of protein to below the detection limit (1 mU/mg). Butyrate no longer can be formed, and the metabolic flux may be diverted to butanol. Butyrate kinase showed a 2.5- to 10-fold increase in specific activity after phosphate butyryltransferase activity no longer could be detected. These results suggest that the uptake of acetate and butyrate during solvent formation can not proceed via a complete reversal of the phosphate transferase and kinase reactions. The activities of all enzymes investigated as a function of time in vitro are much higher than the metabolic fluxes through them in vivo. This indicates that none of the maximal activities of the enzymes assayed is rate limiting in C. acetobutylicum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEESCH S. C. Acetone-butanol fermentation of starches. Appl Microbiol. 1953 Mar;1(2):85–95. doi: 10.1128/am.1.2.85-95.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMEYER H. U., HOLZ G., KLOTZSCH H., LANG G. PHOSPHOTRANSACETYLASE AUS CLOSTRIDIUM KLUYVERI. ZUECHTUNG DES BACTERIUMS, ISOLIERUNG, KRISTALLISATION UND EIGENSCHAFTEN DES ENZYMS. Biochem Z. 1963;338:114–121. [PubMed] [Google Scholar]
- Berndt H., Schlegel H. G. Kinetics and properties of beta-ketothiolase from Clostridium pasteurianum. Arch Microbiol. 1975 Mar 12;103(1):21–30. doi: 10.1007/BF00436325. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davies R., Stephenson M. Studies on the acetone-butyl alcohol fermentation: Nutritional and other factors involved in the preparation of active suspensions of Cl. acetobutylicum (Weizmann). Biochem J. 1941 Dec;35(12):1320–1331. doi: 10.1042/bj0351320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. Studies on the acetone-butyl alcohol fermentation: Intermediates in the fermentation of glucose by Cl. acetobutylicum. 3. Potassium as an essential factor in the fermentation of maize meal by Cl. acetobutylicum (BY). Biochem J. 1942 Sep;36(7-9):582–599. doi: 10.1042/bj0360582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommes V., Kunau W. H. A convenient assay for acyl-CoA-dehydrogenases. Anal Biochem. 1976 Apr;71(2):571–578. doi: 10.1016/s0003-2697(76)80026-7. [DOI] [PubMed] [Google Scholar]
- GAVARD R., HAUTECOEUR B., DESCOURTIEUX H. Phosphotransbutyrylase de Clostridium acetobutylicum. C R Hebd Seances Acad Sci. 1957 Apr 29;244(18):2323–2326. [PubMed] [Google Scholar]
- Gottschalk G., Bahl H. Feasible improvements of the butanol production by Clostridium acetobutylicum. Basic Life Sci. 1981;18:463–471. doi: 10.1007/978-1-4684-3980-9_27. [DOI] [PubMed] [Google Scholar]
- Hartmanis M. G., Stadtman T. C. Isolation of a selenium-containing thiolase from Clostridium kluyveri: identification of the selenium moiety as selenomethionine. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4912–4916. doi: 10.1073/pnas.79.16.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell D. B., Peck M. W., Rodger G., Morris J. G. On the permeability to weak acids and bases of the cytoplasmic membrane of Clostridium pasteurianum. Biochem Biophys Res Commun. 1981 Mar 16;99(1):81–88. doi: 10.1016/0006-291x(81)91715-0. [DOI] [PubMed] [Google Scholar]
- LYNEN F., OCHOA S. Enzymes of fatty acid metabolism. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):299–314. doi: 10.1016/0006-3002(53)90149-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madan V. K., Hillmer P., Gottschalk G. Purification and properties of NADP-dependent L(+)-3-hydroxybutyryl-CoA dehydrogenase from Clostridium kluyveri. Eur J Biochem. 1973 Jan 3;32(1):51–56. doi: 10.1111/j.1432-1033.1973.tb02577.x. [DOI] [PubMed] [Google Scholar]
- Petitdemange H., Cherrier C., Bengone J. M., Gay R. Etude des activités NADH et NADPH-ferrédoxine oxydoréductasiques chez Clostridium acetobutylicum. Can J Microbiol. 1977 Feb;23(2):152–160. [PubMed] [Google Scholar]
- Petitdemange H., Desbordes J., Berthelin J., Gay R. Conversion enzymatique du n-butyraldéhyde en n-butanol chez Clostridium acétobutylicum. C R Acad Sci Hebd Seances Acad Sci D. 1968 Apr 22;266(17):1772–1774. [PubMed] [Google Scholar]
- Petitdemange H., Desbordes J., Maugras M., Gay R. Etude de la formation du n-butanol chez Clostridium acetobutylicum. Bull Soc Chim Biol (Paris) 1969 Jun 4;51(1):157–165. [PubMed] [Google Scholar]
- ROSENFELD B., SIMON E. The mechanism of the butanol-acetone fermentation. I. The rôle of pyruvate as an intermediate. J Biol Chem. 1950 Sep;186(1):395–404. [PubMed] [Google Scholar]
- ROSS D. The acetone-butanol fermentation. Prog Ind Microbiol. 1961;3:71–90. [PubMed] [Google Scholar]
- Riebeling V., Thauer R. K., Jungermann K. The internal-alkaline pH gradient, sensitive to uncoupler and ATPase inhibitor, in growing Clostridium pasteurianum. Eur J Biochem. 1975 Jul 1;55(2):445–453. doi: 10.1111/j.1432-1033.1975.tb02181.x. [DOI] [PubMed] [Google Scholar]
- SLY W. S., STADTMAN E. R. FORMATE METABOLISM. II. ENZYMATIC SYNTHESIS OF FORMYL PHOSPHATE AND FORMYL COENZYME A IN CLOSTRIDIUM CYLINDROSPORUM. J Biol Chem. 1963 Aug;238:2639–2647. [PubMed] [Google Scholar]
- STADTMAN E. R., BARKER H. A. Fatty acid synthesis by enzyme preparations of Clostridium kluyveri. VI. Reactions of acyl phosphates. J Biol Chem. 1950 Jun;184(2):769–793. [PubMed] [Google Scholar]
- STADTMAN E. R. The purification and properties of phosphotransacetylase. J Biol Chem. 1952 May;196(2):527–534. [PubMed] [Google Scholar]
- STERN J. R. Optical properties of aceto-acetyl-S-coenzyme A and its metal chelates. J Biol Chem. 1956 Jul;221(1):33–44. [PubMed] [Google Scholar]
- TWAROG R., WOLFE R. S. Enzymatic phosphorylation of butyrate. J Biol Chem. 1962 Aug;237:2474–2477. [PubMed] [Google Scholar]
- TWAROG R., WOLFE R. S. ROLE OF BUTYRYL PHOSPHATE IN THE ENERGY METABOLISM OF CLOSTRIDIUM TETANOMORPHUM. J Bacteriol. 1963 Jul;86:112–117. doi: 10.1128/jb.86.1.112-117.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C., WOLFE R. S. Purification and role of phosphotransbutyrylase. J Biol Chem. 1960 Jul;235:1948–1952. [PubMed] [Google Scholar]
- Waterson R. M., Castellino F. J., Hass G. M., Hill R. L. Purification and characterization of crotonase from Clostridium acetobutylicum. J Biol Chem. 1972 Aug 25;247(16):5266–5271. [PubMed] [Google Scholar]
- Weyer E. R., Rettger L. F. A COMPARATIVE STUDY OF SIX DIFFERENT STRAINS OF THE ORGANISM COMMONLY CONCERNED IN LARGE-SCALE PRODUCTION OF BUTYL ALCOHOL AND ACETONE BY THE BIOLOGICAL PROCESS. J Bacteriol. 1927 Dec;14(6):399–424. doi: 10.1128/jb.14.6.399-424.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerner B., Coutts S. M., Lederer F., Waters H. H., Westheimer F. H. Acetoacetate decarboxylase. Preparation of the enzyme. Biochemistry. 1966 Mar;5(3):813–816. doi: 10.1021/bi00867a001. [DOI] [PubMed] [Google Scholar]
- von Hugo H., Schoberth S., Madan V. K., Gottschalk G. Coenzyme specificity of dehydrogenases and fermentation of pyruvate by clostridia. Arch Mikrobiol. 1972;87(3):189–202. doi: 10.1007/BF00424880. [DOI] [PubMed] [Google Scholar]


