Abstract
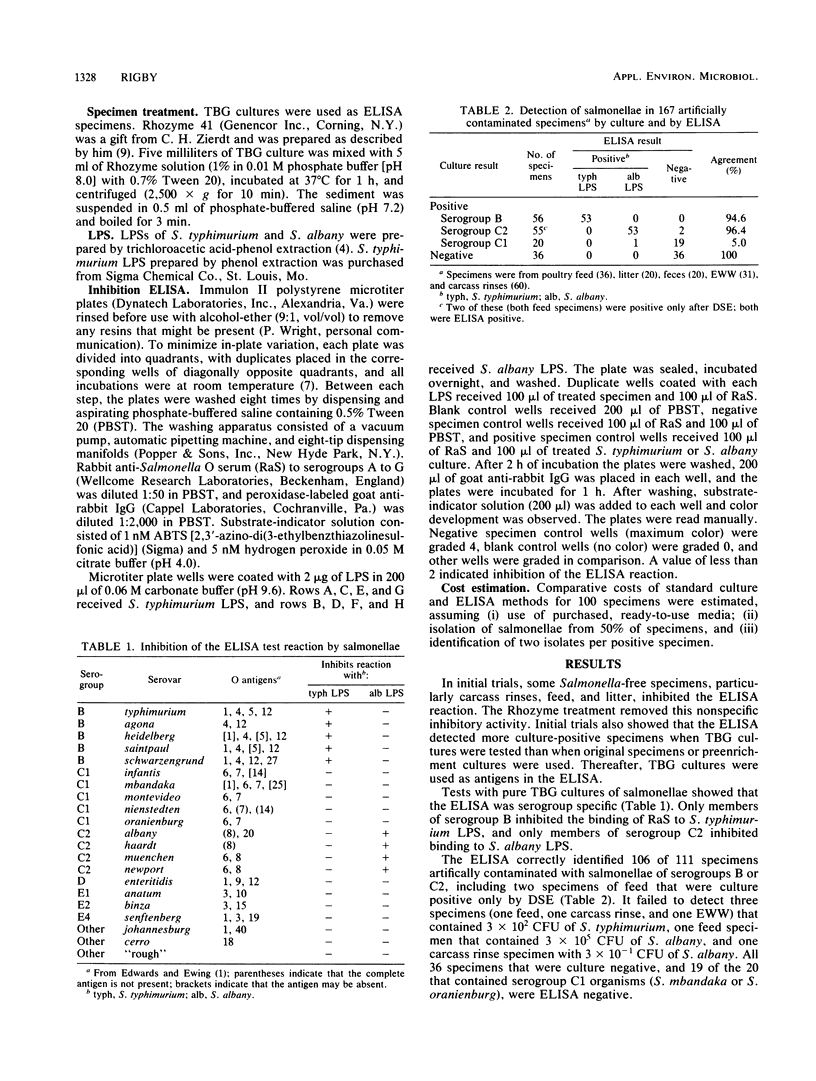
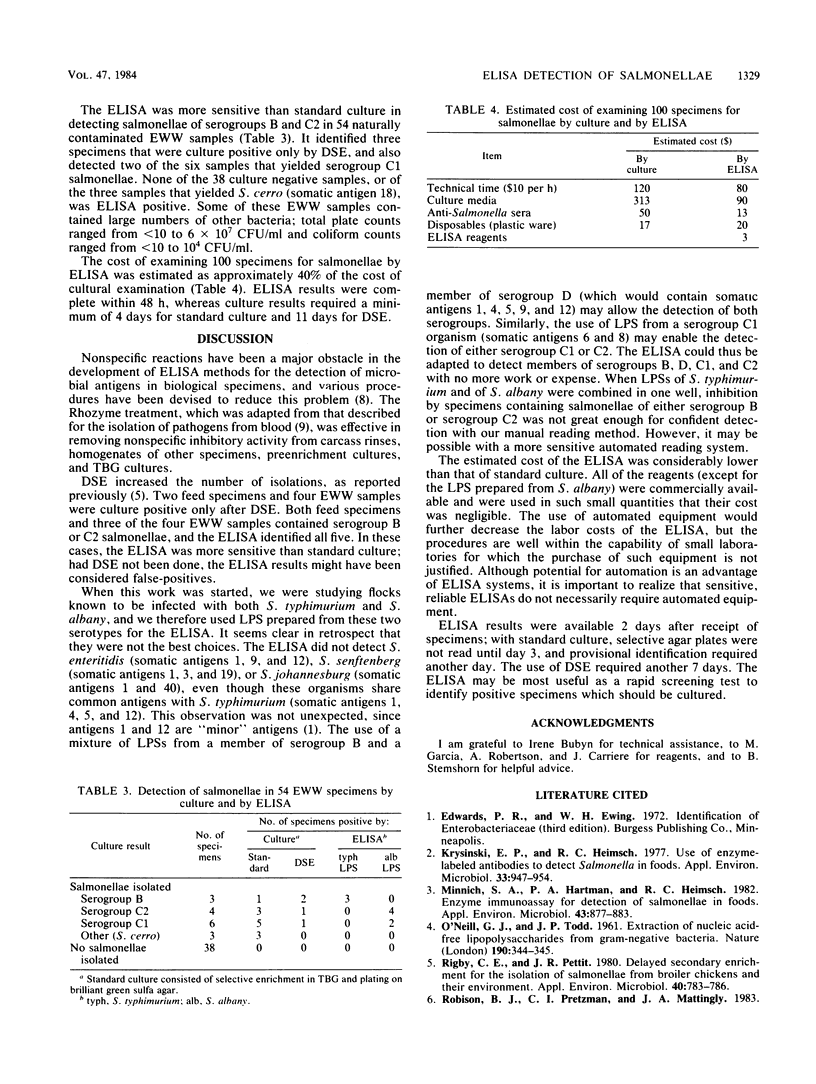
An enzyme-linked immunosorbent assay (ELISA) for detection of salmonellae was developed and evaluated by using artificially contaminated specimens of poultry feed, feces, litter, or carcass rinsings, and naturally contaminated water samples. Specimens containing salmonellae of serogroups B or C2 inhibited the binding of polyvalent anti-O serum to microtiter plate wells coated with lipopolysaccharide of Salmonella typhimurium (serogroup B) or Salmonella albany (serogroup C2), respectively. Treatment of specimens with Rhozyme 41 (a protease) inhibited nonspecific reactions. The ELISA detected 106 of 111 culture-positive specimens contaminated with salmonellae of serogroups B or C2. Nineteen of 20 specimens containing salmonellae of serogroup C1 and all of 36 culture-negative specimens were ELISA negative. All seven water samples that contained salmonellae of serogroups B or C2, including three that were culture positive only after delayed secondary enrichment, were ELISA positive. Seven of the nine water samples that contained salmonellae of other serogroups, and all 38 culture-negative samples, were ELISA negative. The ELISA was simple to perform, produced results in 48 h, and was more economical than culture methods.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Krysinski E. P., Heimsch R. C. Use of enzyme-labeled antibodies to detect Salmonella in foods. Appl Environ Microbiol. 1977 Apr;33(4):947–954. doi: 10.1128/aem.33.4.947-954.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnich S. A., Hartman P. A., Heimsch R. C. Enzyme immunoassay for detection of Salmonellae in foods. Appl Environ Microbiol. 1982 Apr;43(4):877–893. doi: 10.1128/aem.43.4.877-893.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'NEILL G. J., TODD J. P. Extraction of nucleic acid-free lipopolysaccharides from gram-negative bacteria. Nature. 1961 Apr 22;190:344–345. doi: 10.1038/190344b0. [DOI] [PubMed] [Google Scholar]
- Rigby C. E., Pettit J. R. Delayed secondary enrichment for the isolation of salmonellae from broiler chickens and their environment. Appl Environ Microbiol. 1980 Oct;40(4):783–786. doi: 10.1128/aem.40.4.783-786.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemshorn B. W., Buckley D. J., St Amour G., Lin C. S., Duncan J. R. A computer-interfaced photometer and systematic spacing of duplicates to control within-plate enzyme-immunoassay variation. J Immunol Methods. 1983 Jul 29;61(3):367–375. doi: 10.1016/0022-1759(83)90233-8. [DOI] [PubMed] [Google Scholar]
- Yolken R. H. Enzyme immunoassays for the detection of infectious antigens in body fluids: current limitations and future prospects. Rev Infect Dis. 1982 Jan-Feb;4(1):35–68. doi: 10.1093/clinids/4.1.35. [DOI] [PubMed] [Google Scholar]
- Zierdt C. H. Blood-lysing solution nontoxic to pathogenic bacteria. J Clin Microbiol. 1982 Jan;15(1):172–174. doi: 10.1128/jcm.15.1.172-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


