Abstract
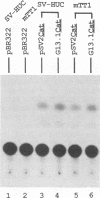
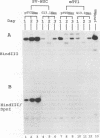
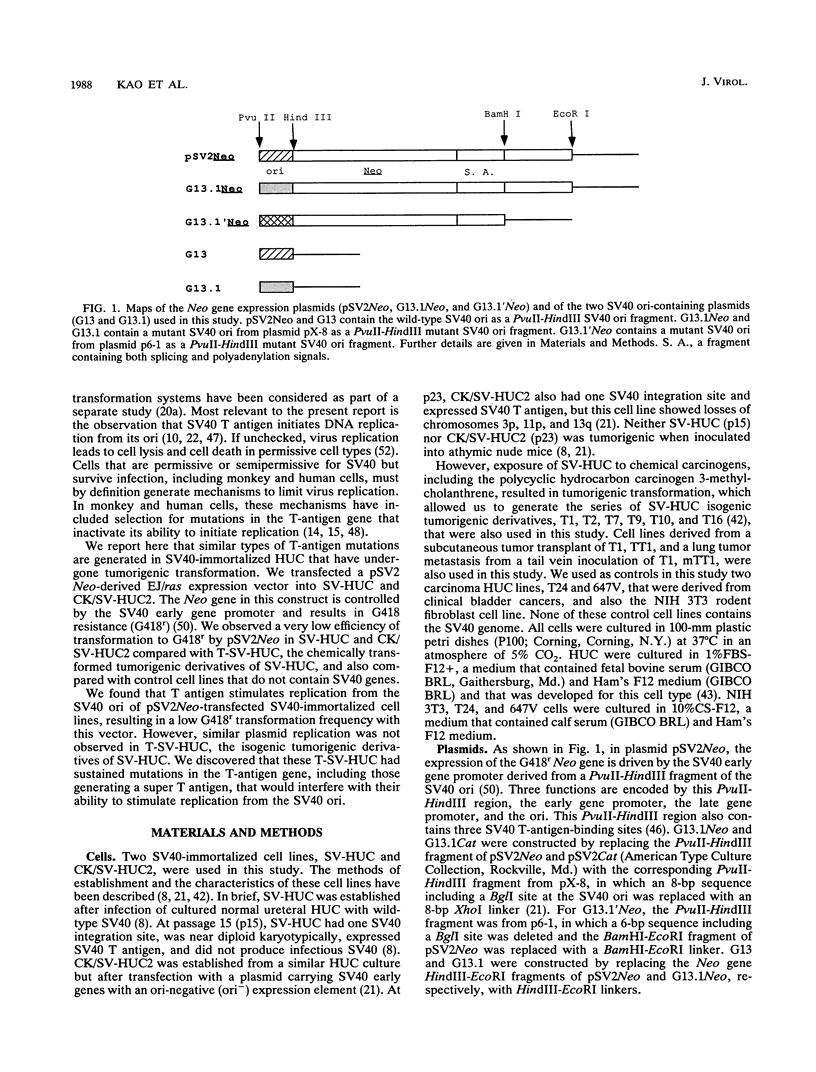
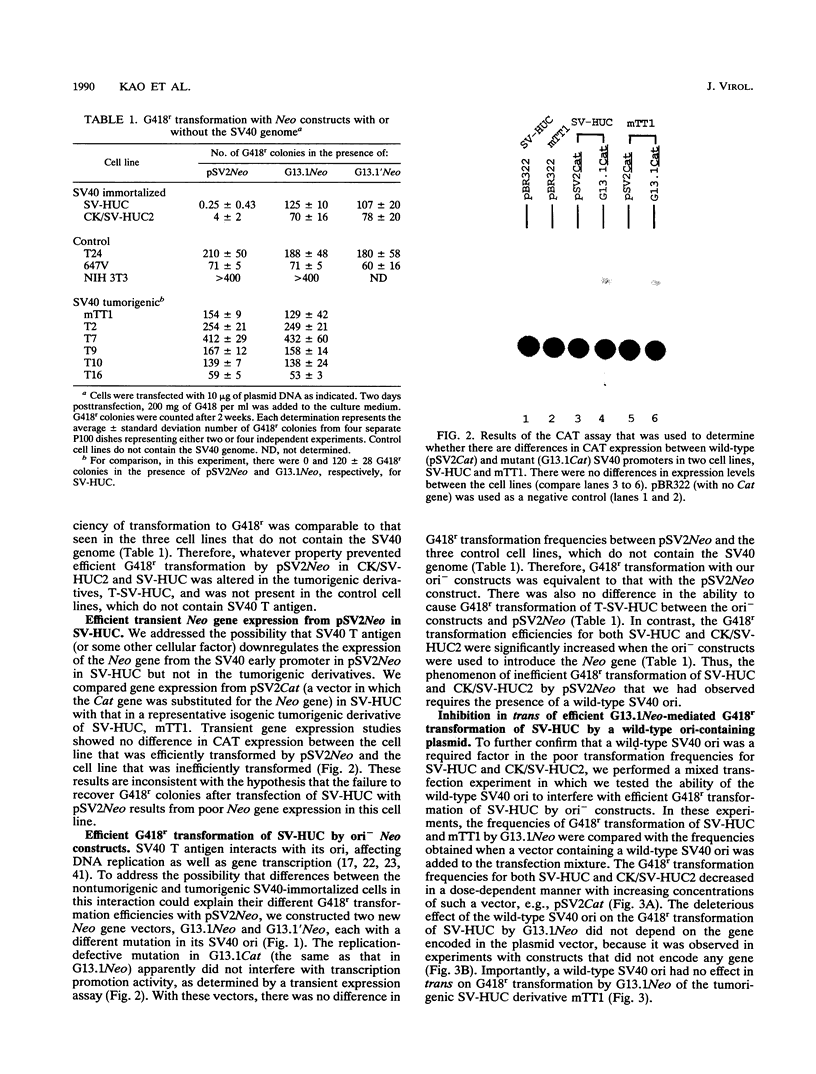
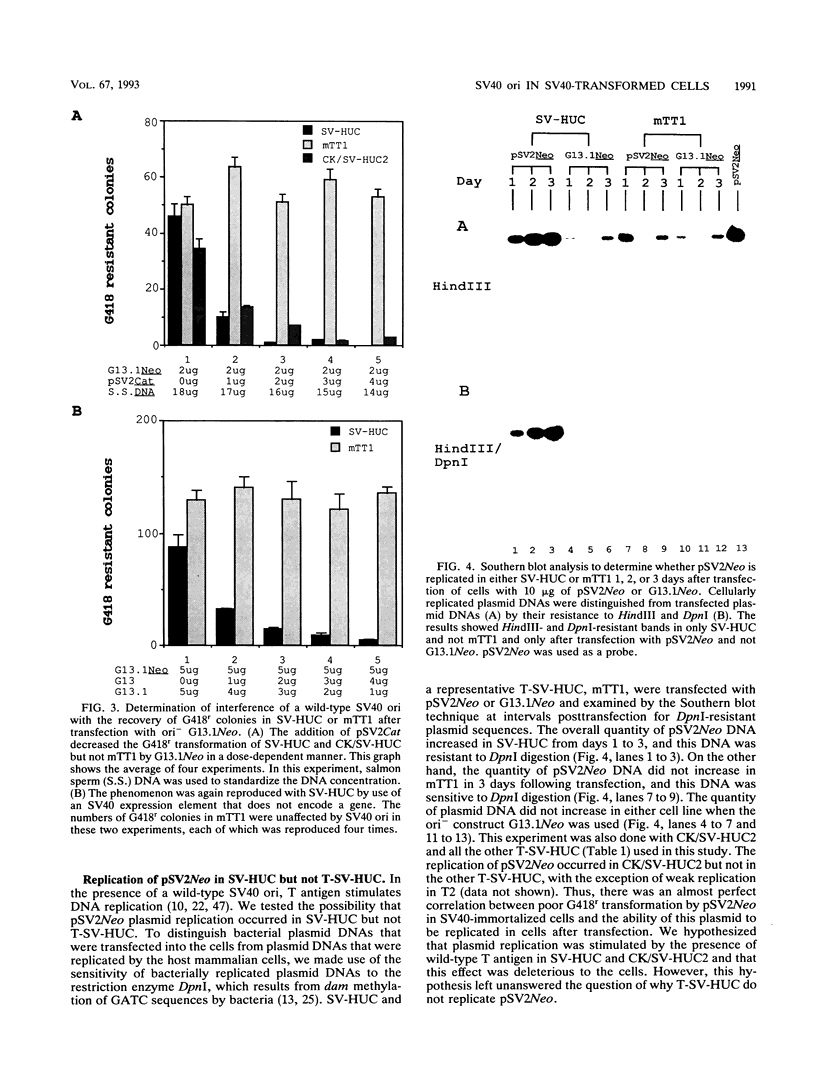
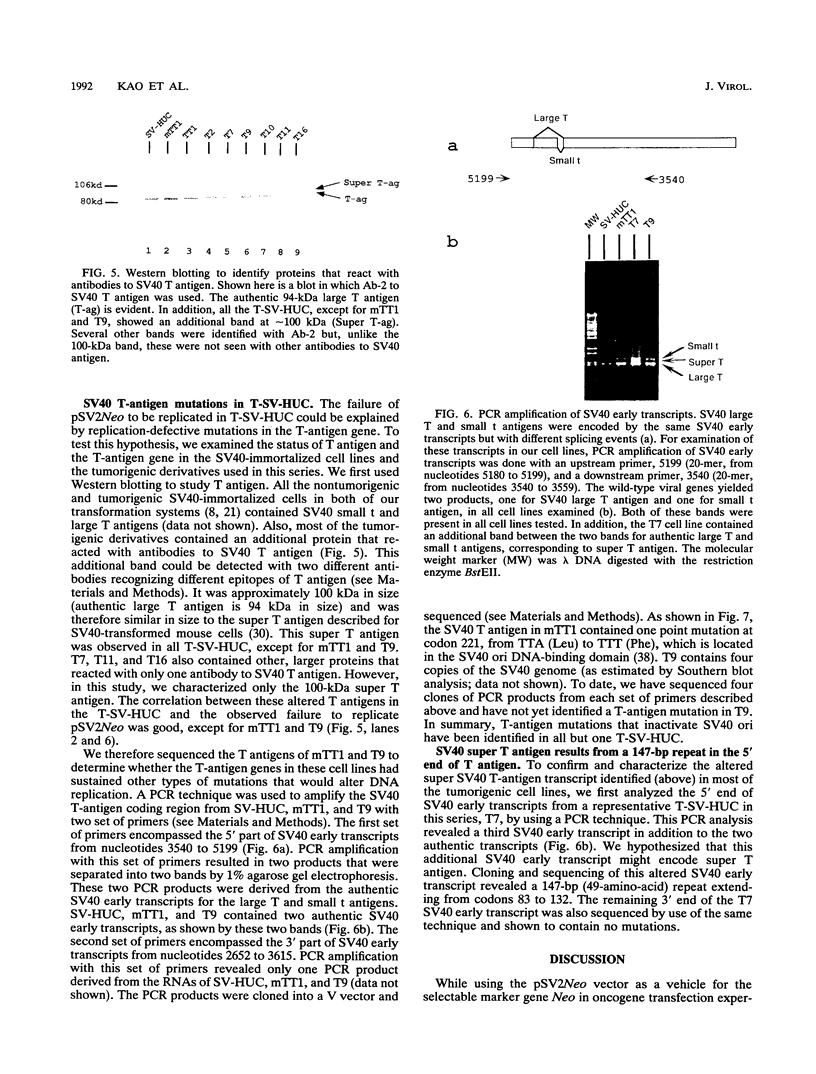
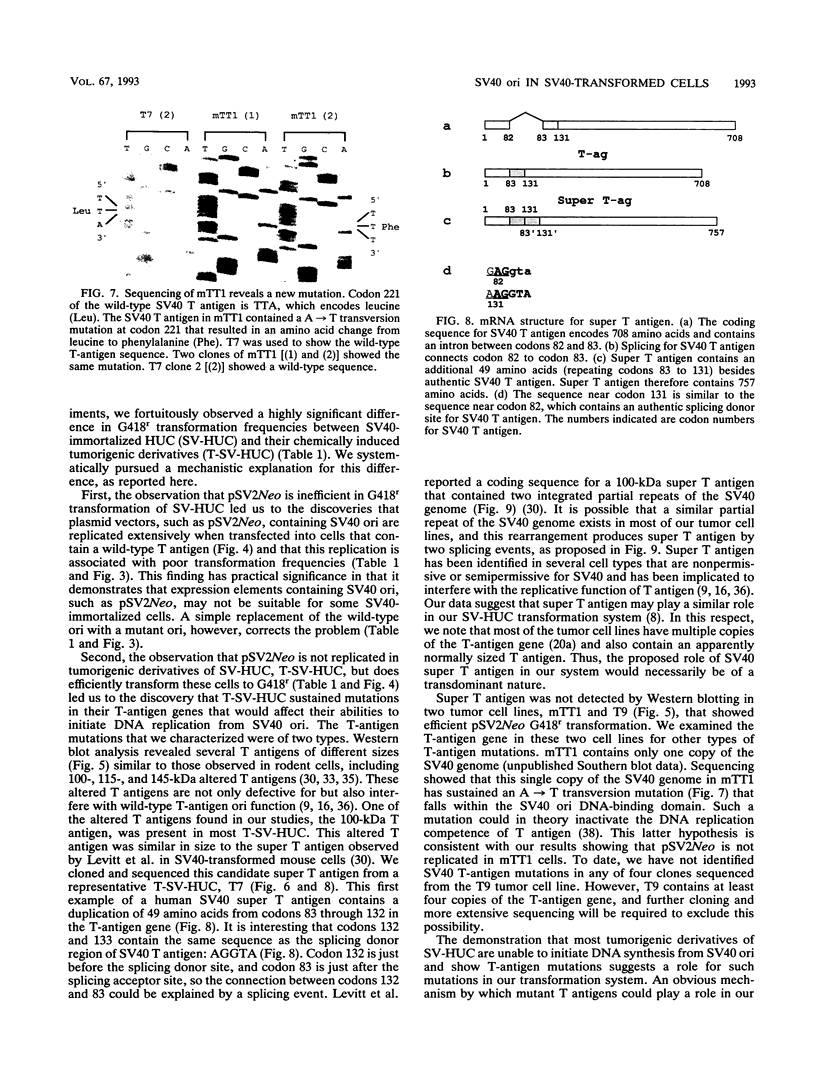
pSV2Neo, a plasmid that contains the wild-type simian virus 40 (SV40) origin of replication (ori), is widely used in mammalian cell transfection experiments. We observed that pSV2Neo transforms two nontumorigenic SV40-immortalized human uroepithelial cell lines (SV-HUC and CK/SV-HUC2) to G418 resistance (G418r) at a frequency lower than that at which it transforms SV-HUC tumorigenic derivatives (T-SV-HUC). Transient expression studies with the chloramphenicol transferase assay showed that these differences could not be explained by differences in Neo gene expression. However, when we replaced the SV40 ori in pSV2Neo with a replication-defective ori to generate G13.1Neo and G13.1'Neo, the G418r transformation frequency of the SV40-immortalized cell lines was elevated. Because SV40 T antigen stimulates replication at its ori, we tested plasmid replication in these transfected cell lines. The immortalized cell lines that showed low G418r transformation frequencies after transfection with pSV2Neo showed high levels of plasmid replication, while the T-SV-HUC that showed high G418r transformation frequencies failed to replicate pSV2Neo. To determine whether differences in the status of the T-antigen gene contributed to the phenomenon, we characterized the T-antigen gene in these cell lines. The results showed that the T-SV-HUC had sustained mutations in the T-antigen gene that would interfere with the ability of the T antigen to stimulate replication at its ori. Most T-SV-HUC contained a super-T-antigen replication-defective ori that apparently resulted from the partial duplication of SV40 early genes, but one T-SV-HUC had a point mutation in the ori DNA-binding domain of the T-antigen gene. These results correlate with the high G418r transformation frequencies with pSV2Neo in T-SV-HUC compared with SV-HUC and CK/SV-HUC2. Furthermore, these results suggest that alterations in SV40 T antigen may be important in stabilizing human cells immortalized by SV40 genes that contain the wild-type SV40 ori, thus contributing to tumorigenic transformation. This is the first report of a super T antigen occurring in human SV40-transformed cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bookland E. A., Reznikoff C. A., Lindstrom M., Swaminathan S. Induction of thioguanine-resistant mutations in human uroepithelial cells by 4-aminobiphenyl and its N-hydroxy derivatives. Cancer Res. 1992 Mar 15;52(6):1615–1621. [PubMed] [Google Scholar]
- Bookland E. A., Swaminathan S., Oyasu R., Gilchrist K. W., Lindstrom M., Reznikoff C. A. Tumorigenic transformation and neoplastic progression of human uroepithelial cells after exposure in vitro to 4-aminobiphenyl or its metabolites. Cancer Res. 1992 Mar 15;52(6):1606–1614. [PubMed] [Google Scholar]
- Bradley M. K., Smith T. F., Lathrop R. H., Livingston D. M., Webster T. A. Consensus topography in the ATP binding site of the simian virus 40 and polyomavirus large tumor antigens. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4026–4030. doi: 10.1073/pnas.84.12.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Jarvis D. L. The plasma-membrane-associated form of SV40 large tumor antigen: biochemical and biological properties. Biochim Biophys Acta. 1986 Oct 28;865(2):171–195. doi: 10.1016/0304-419x(86)90027-2. [DOI] [PubMed] [Google Scholar]
- Chang S. E. In vitro transformation of human epithelial cells. Biochim Biophys Acta. 1986;823(3):161–194. doi: 10.1016/0304-419x(86)90001-6. [DOI] [PubMed] [Google Scholar]
- Chang S. E., Keen J., Lane E. B., Taylor-Papadimitriou J. Establishment and characterization of SV40-transformed human breast epithelial cell lines. Cancer Res. 1982 May;42(5):2040–2053. [PubMed] [Google Scholar]
- Christian B. J., Kao C. H., Wu S. Q., Meisner L. F., Reznikoff C. A. EJ/ras neoplastic transformation of simian virus 40-immortalized human uroepithelial cells: a rare event. Cancer Res. 1990 Aug 1;50(15):4779–4786. [PubMed] [Google Scholar]
- Christian B. J., Loretz L. J., Oberley T. D., Reznikoff C. A. Characterization of human uroepithelial cells immortalized in vitro by simian virus 40. Cancer Res. 1987 Nov 15;47(22):6066–6073. [PubMed] [Google Scholar]
- Clayton C. E., Lovett M., Rigby P. W. Functional analysis of a simian virus 40 super T-antigen. J Virol. 1982 Dec;44(3):974–982. doi: 10.1128/jvi.44.3.974-982.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S. E., Liu C. P., Botchan M. R. Fragment spanning the SV40 replication origin is the only DNA sequence required in cis for viral excision. Science. 1982 Dec 17;218(4578):1223–1225. doi: 10.1126/science.6293055. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DiPaolo J. A. Relative difficulties in transforming human and animal cells in vitro. J Natl Cancer Inst. 1983 Jan;70(1):3–8. [PubMed] [Google Scholar]
- Geier G. E., Modrich P. Recognition sequence of the dam methylase of Escherichia coli K12 and mode of cleavage of Dpn I endonuclease. J Biol Chem. 1979 Feb 25;254(4):1408–1413. [PubMed] [Google Scholar]
- Gish W. R., Botchan M. R. Simian virus 40-transformed human cells that express large T antigens defective for viral DNA replication. J Virol. 1987 Sep;61(9):2864–2876. doi: 10.1128/jvi.61.9.2864-2876.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Davison J., Oren M., Winocour E. Properties of permissive monkey cells transformed by UV-irradiated simian virus 40. J Virol. 1977 May;22(2):256–266. doi: 10.1128/jvi.22.2.256-266.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen U., Tenen D. G., Livingston D. M., Sharp P. A. T antigen repression of SV40 early transcription from two promoters. Cell. 1981 Dec;27(3 Pt 2):603–613. doi: 10.1016/0092-8674(81)90402-5. [DOI] [PubMed] [Google Scholar]
- Harris C. C. Human tissues and cells in carcinogenesis research. Cancer Res. 1987 Jan 1;47(1):1–10. [PubMed] [Google Scholar]
- Kahn P., Topp W. C., Shin S. Tumorigenicity of SV40-transformed human and monkey cells in immunodeficient mice. Virology. 1983 Apr 15;126(1):348–360. doi: 10.1016/0042-6822(83)90484-1. [DOI] [PubMed] [Google Scholar]
- Kaighn M. E., Reddel R. R., Lechner J. F., Peehl D. M., Camalier R. F., Brash D. E., Saffiotti U., Harris C. C. Transformation of human neonatal prostate epithelial cells by strontium phosphate transfection with a plasmid containing SV40 early region genes. Cancer Res. 1989 Jun 1;49(11):3050–3056. [PubMed] [Google Scholar]
- Kao C., Wu S. Q., Bhatthacharya M., Meisner L. F., Reznikoff C. A. Losses of 3p, 11p, and 13q in EJ/ras-transformable simian virus 40-immortalized human uroepithelial cells. Genes Chromosomes Cancer. 1992 Mar;4(2):158–168. doi: 10.1002/gcc.2870040210. [DOI] [PubMed] [Google Scholar]
- Kelly T. J. SV40 DNA replication. J Biol Chem. 1988 Dec 5;263(34):17889–17892. [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. A deoxyribonuclease of Diplococcus pneumoniae specific for methylated DNA. J Biol Chem. 1975 Jun 10;250(11):4060–4066. [PubMed] [Google Scholar]
- Lambert M. E., Pellegrini S., Gattoni-Celli S., Weinstein I. B. Carcinogen induced asynchronous replication of polyoma DNA is mediated by a trans-acting factor. Carcinogenesis. 1986 Jun;7(6):1011–1017. doi: 10.1093/carcin/7.6.1011. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lavi S. Carcinogen-mediated amplification of viral DNA sequences in simian virus 40-transformed Chinese hamster embryo cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6144–6148. doi: 10.1073/pnas.78.10.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine N. R., Mayall E. S., Jones T., Sheer D., McDermid S., Kendall-Taylor P., Wynford-Thomas D. Characterisation of human thyroid epithelial cells immortalised in vitro by simian virus 40 DNA transfection. Br J Cancer. 1989 Dec;60(6):897–903. doi: 10.1038/bjc.1989.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt A., Chen S., Blanck G., George D., Pollack R. E. Two integrated partial repeats of simian virus 40 together code for a super-T antigen. Mol Cell Biol. 1985 Apr;5(4):742–750. doi: 10.1128/mcb.5.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Bradley M. K. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987 Apr;4(2):63–80. [PubMed] [Google Scholar]
- Lovett M., Clayton C. E., Murphy D., Rigby P. W., Smith A. E., Chaudry F. Structure and synthesis of a simian virus 40 super T-antigen. J Virol. 1982 Dec;44(3):963–973. doi: 10.1128/jvi.44.3.963-973.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., Jeltsch J. M., Gannon F. Characterization of a gene encoding a 115 K super T antigen expressed by a SV40-transformed rat cell line. Nucleic Acids Res. 1981 Aug 25;9(16):4111–4128. doi: 10.1093/nar/9.16.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., Lasne C., Prives C., Borde J., May P. Study of the functional activities concomitantly retained by the 115,000 Mr super T antigen, an evolutionary variant of simian virus 40 large T antigen expressed in transformed rat cells. J Virol. 1983 Mar;45(3):901–913. doi: 10.1128/jvi.45.3.901-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero J. A., Stitt D. T., Mangel W. F., Carroll R. B. Identification of new polypeptide species (48-55K) immunoprecipitable by antiserum to purified large T antigen and present in SV40-infected and -transformed cells. Virology. 1979 Mar;93(2):466–480. doi: 10.1016/0042-6822(79)90250-2. [DOI] [PubMed] [Google Scholar]
- Paucha E., Kalderon D., Harvey R. W., Smith A. E. Simian virus 40 origin DNA-binding domain on large T antigen. J Virol. 1986 Jan;57(1):50–64. doi: 10.1128/jvi.57.1.50-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt C. I., Kao C. H., Wu S. Q., Gilchrist K. W., Oyasu R., Reznikoff C. A. Neoplastic progression by EJ/ras at different steps of transformation in vitro of human uroepithelial cells. Cancer Res. 1992 Feb 1;52(3):688–695. [PubMed] [Google Scholar]
- Reddel R. R., Ke Y., Gerwin B. I., McMenamin M. G., Lechner J. F., Su R. T., Brash D. E., Park J. B., Rhim J. S., Harris C. C. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988 Apr 1;48(7):1904–1909. [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff C. A., Loretz L. J., Christian B. J., Wu S. Q., Meisner L. F. Neoplastic transformation of SV40-immortalized human urinary tract epithelial cells by in vitro exposure to 3-methylcholanthrene. Carcinogenesis. 1988 Aug;9(8):1427–1436. doi: 10.1093/carcin/9.8.1427. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Loretz L. J., Pesciotta D. M., Oberley T. D., Ignjatovic M. M. Growth kinetics and differentiation in vitro of normal human uroepithelial cells on collagen gel substrates in defined medium. J Cell Physiol. 1987 Jun;131(3):285–301. doi: 10.1002/jcp.1041310302. [DOI] [PubMed] [Google Scholar]
- Rhim J. S. Neoplastic transformation of human epithelial cells in vitro. Anticancer Res. 1989 Sep-Oct;9(5):1345–1365. [PubMed] [Google Scholar]
- Scheffner M., Knippers R., Stahl H. RNA unwinding activity of SV40 large T antigen. Cell. 1989 Jun 16;57(6):955–963. doi: 10.1016/0092-8674(89)90334-6. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Analysis of mechanisms controlling the interactions of SV40 large T antigen with the SV40 ORI region. Virology. 1988 Aug;165(2):527–538. doi: 10.1016/0042-6822(88)90597-1. [DOI] [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H. Transformation of green monkey kidney cells by SV40 genome: the establishment of transformed cell lines and the replication of human adenoviruses and SV40 in transformed cells. Virology. 1971 Jul;45(1):163–171. doi: 10.1016/0042-6822(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986 Nov;6(11):4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stahl H., Knippers R. The simian virus 40 large tumor antigen. Biochim Biophys Acta. 1987 Oct 9;910(1):1–10. doi: 10.1016/0167-4781(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Wiekowski M., Schwarz M. W., Stahl H. Simian virus 40 large T antigen DNA helicase. Characterization of the ATPase-dependent DNA unwinding activity and its substrate requirements. J Biol Chem. 1988 Jan 5;263(1):436–442. [PubMed] [Google Scholar]
- Wu S. Q., Storer B. E., Bookland E. A., Klingelhutz A. J., Gilchrist K. W., Meisner L. F., Oyasu R., Reznikoff C. A. Nonrandom chromosome losses in stepwise neoplastic transformation in vitro of human uroepithelial cells. Cancer Res. 1991 Jun 15;51(12):3323–3326. [PubMed] [Google Scholar]