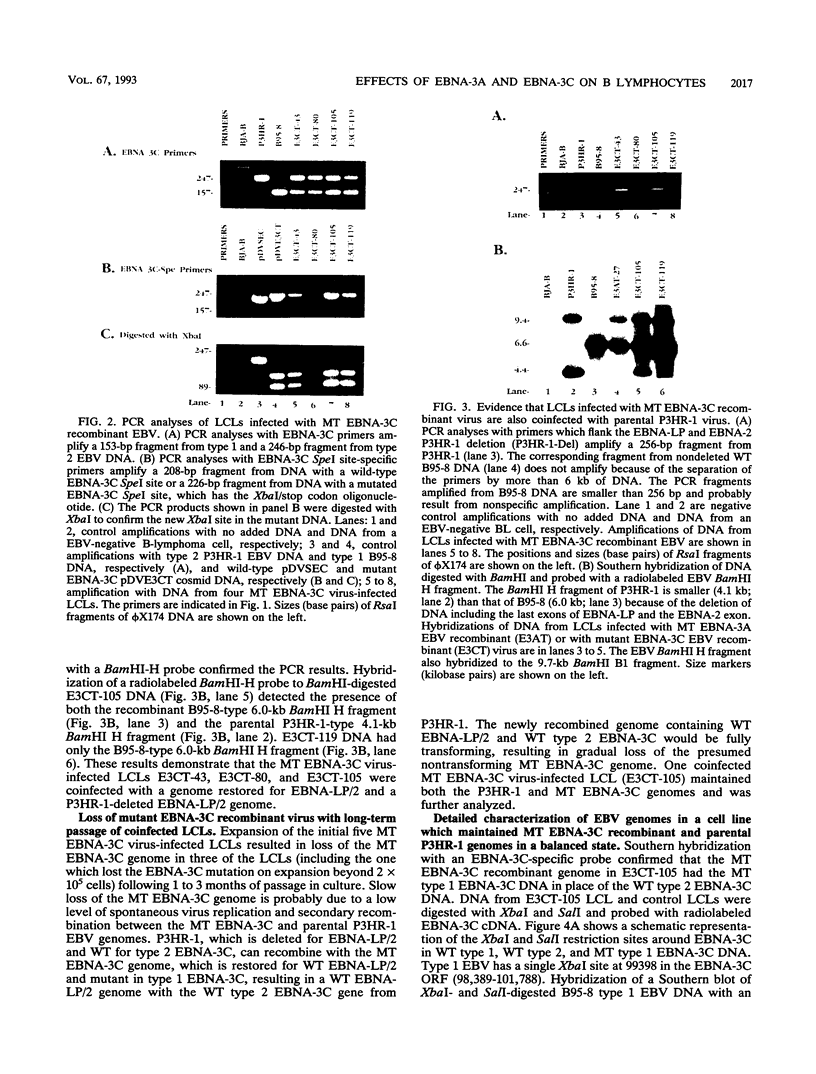
Abstract
Recombinant Epstein-Barr viruses (EBV) with a translation termination codon mutation inserted into the nuclear protein 3A (EBNA-3A) or 3C (EBNA-3C) open reading frame were generated by second-site homologous recombination. These mutant viruses were used to infect primary B lymphocytes to assess the requirement of EBNA-3A or -3C for growth transformation. The frequency of obtaining transformants infected with a wild-type EBNA-3A recombinant EBV was 10 to 15%. In contrast, the frequency of obtaining transformants infected with a mutant EBNA-3A recombinant EBV was only 1.4% (9 mutants in 627 transformants analyzed). Transformants infected with mutant EBNA-3A recombinant virus could be obtained only by coinfection with another transformation-defective EBV which provided wild-type EBNA-3A in trans. Cells infected with mutant EBNA-3A recombinant virus lost the EBNA-3A mutation with expansion of the culture. The decreased frequency of recovery of the EBNA-3A mutation, the requirement for transformation-defective EBV coinfection, and the inability to maintain the EBNA-3A mutation indicate that EBNA-3A is essential or critical for lymphocyte growth transformation and that the EBNA-3A mutation has a partial dominant negative effect. Five transformants infected with mutant EBNA-3C recombinant virus EBV were also identified and expanded. All five also required wild-type EBNA-3C in trans. Serial passage of the mutant recombinant virus into primary B lymphocytes resulted in transformants only when wild-type EBNA-3C was provided in trans by coinfection with a transformation-defective EBV carrying a wild-type EBNA-3C gene. A secondary recombinant virus in which the mutated EBNA-3C gene was replaced by wild-type EBNA-3C was able to transform B lymphocytes. Thus, EBNA-3C is also essential or critical for primary B-lymphocyte growth transformation.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adldinger H. K., Delius H., Freese U. K., Clarke J., Bornkamm G. W. A putative transforming gene of Jijoye virus differs from that of Epstein-Barr virus prototypes. Virology. 1985 Mar;141(2):221–234. doi: 10.1016/0042-6822(85)90253-3. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos I., Herbst H., Niedobitek G., Stein H. Demonstration of monoclonal EBV genomes in Hodgkin's disease and Ki-1-positive anaplastic large cell lymphoma by combined Southern blot and in situ hybridization. Blood. 1989 Aug 1;74(2):810–816. [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bird A. G., Britton S., Ernberg I., Nilsson K. Characteristics of Epstein-Barr virus activation of human B lymphocytes. J Exp Med. 1981 Sep 1;154(3):832–839. doi: 10.1084/jem.154.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., Hudewentz J., Freese U. K., Zimber U. Deletion of the nontransforming Epstein-Barr virus strain P3HR-1 causes fusion of the large internal repeat to the DSL region. J Virol. 1982 Sep;43(3):952–968. doi: 10.1128/jvi.43.3.952-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Kieff E. Epstein-Barr virus nuclear protein 2 mutations define essential domains for transformation and transactivation. J Virol. 1991 May;65(5):2545–2554. doi: 10.1128/jvi.65.5.2545-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Mannick J., Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Jenson H., Seibl R., Wolf H., Miller G. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J Virol. 1987 Dec;61(12):3672–3679. doi: 10.1128/jvi.61.12.3672-3679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges C., Wolf H., De-Thé G., Shanmugaratnam K., Cammoun N., Ellouz R., Klein G., Lennert K., Muñoz N., Zur Hausen H. Nasopharyngeal carcinoma. X. Presence of epstein-barr genomes in separated epithelial cells of tumours in patients from Singapore, Tunisia and Kenya. Int J Cancer. 1975 Jul 15;16(1):7–15. doi: 10.1002/ijc.2910160103. [DOI] [PubMed] [Google Scholar]
- Geballe A. P., Mocarski E. S. Translational control of cytomegalovirus gene expression is mediated by upstream AUG codons. J Virol. 1988 Sep;62(9):3334–3340. doi: 10.1128/jvi.62.9.3334-3340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Walker L., Guy G., Brown G., Rowe M., Rickinson A. Control of human B-lymphocyte replication. II. Transforming Epstein-Barr virus exploits three distinct viral signals to undermine three separate control points in B-cell growth. Immunology. 1986 Aug;58(4):591–595. [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Heller M., Dambaugh T., Kieff E. Epstein-Barr virus DNA. IX. Variation among viral DNAs from producer and nonproducer infected cells. J Virol. 1981 May;38(2):632–648. doi: 10.1128/jvi.38.2.632-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Ho H. C., Burtin P., Cachin Y., Clifford P., de Schryver A., de-Thé G., Diehl V., Klein G. Antibodies to Epstein-Barr virus in nasopharyngeal carcinoma, other head and neck neoplasms, and control groups. J Natl Cancer Inst. 1970 Jan;44(1):225–231. [PubMed] [Google Scholar]
- Hennessy K., Kieff E. A second nuclear protein is encoded by Epstein-Barr virus in latent infection. Science. 1985 Mar 8;227(4691):1238–1240. doi: 10.1126/science.2983420. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Wang F., Bushman E. W., Kieff E. Definitive identification of a member of the Epstein-Barr virus nuclear protein 3 family. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5693–5697. doi: 10.1073/pnas.83.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Heston L., Rabson M., Brown N., Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982 Jan 14;295(5845):160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- Jeang K. T., Hayward S. D. Organization of the Epstein-Barr virus DNA molecule. III. Location of the P3HR-1 deletion junction and characterization of the NotI repeat units that form part of the template for an abundant 12-O-tetradecanoylphorbol-13-acetate-induced mRNA transcript. J Virol. 1983 Oct;48(1):135–148. doi: 10.1128/jvi.48.1.135-148.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joab I., Rowe D. T., Bodescot M., Nicolas J. C., Farrell P. J., Perricaudet M. Mapping of the gene coding for Epstein-Barr virus-determined nuclear antigen EBNA3 and its transient overexpression in a human cell line by using an adenovirus expression vector. J Virol. 1987 Oct;61(10):3340–3344. doi: 10.1128/jvi.61.10.3340-3344.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdiles B., Walls D., Triki H., Perricaudet M., Joab I. cDNA cloning and transient expression of the Epstein-Barr virus-determined nuclear antigen EBNA3B in human cells and identification of novel transcripts from its coding region. J Virol. 1990 Apr;64(4):1812–1816. doi: 10.1128/jvi.64.4.1812-1816.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W., Dambaugh T., Heller M., Dowling J., Kieff E. Epstein-Barr virus DNA XII. A variable region of the Epstein-Barr virus genome is included in the P3HR-1 deletion. J Virol. 1982 Sep;43(3):979–986. doi: 10.1128/jvi.43.3.979-986.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Hutt L. M., Shaw J. E., Li J. L., Pagano J. S. Replication of EBV in epithelial cells during infectious mononucleosis. Nature. 1977 Jul 21;268(5617):268–270. doi: 10.1038/268268a0. [DOI] [PubMed] [Google Scholar]
- Longnecker R., Miller C. L., Miao X. Q., Marchini A., Kieff E. The only domain which distinguishes Epstein-Barr virus latent membrane protein 2A (LMP2A) from LMP2B is dispensable for lymphocyte infection and growth transformation in vitro; LMP2A is therefore nonessential. J Virol. 1992 Nov;66(11):6461–6469. doi: 10.1128/jvi.66.11.6461-6469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrath I. The pathogenesis of Burkitt's lymphoma. Adv Cancer Res. 1990;55:133–270. doi: 10.1016/s0065-230x(08)60470-4. [DOI] [PubMed] [Google Scholar]
- Mannick J. B., Cohen J. I., Birkenbach M., Marchini A., Kieff E. The Epstein-Barr virus nuclear protein encoded by the leader of the EBNA RNAs is important in B-lymphocyte transformation. J Virol. 1991 Dec;65(12):6826–6837. doi: 10.1128/jvi.65.12.6826-6837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini A., Tomkinson B., Cohen J. I., Kieff E. BHRF1, the Epstein-Barr virus gene with homology to Bc12, is dispensable for B-lymphocyte transformation and virus replication. J Virol. 1991 Nov;65(11):5991–6000. doi: 10.1128/jvi.65.11.5991-6000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci M. G., Szigeti R., Ernberg I., Hu C. P., Torsteinsdottir S., Frade R., Klein E. Activation of B lymphocytes by Epstein-Barr virus/CR2 receptor interaction. Eur J Immunol. 1987 Jun;17(6):815–820. doi: 10.1002/eji.1830170613. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Klein G., Henle W., Henle G. The establishment of lymphoblastoid lines from adult and fetal human lymphoid tissue and its dependence on EBV. Int J Cancer. 1971 Nov 15;8(3):443–450. doi: 10.1002/ijc.2910080312. [DOI] [PubMed] [Google Scholar]
- Petti L., Kieff E. A sixth Epstein-Barr virus nuclear protein (EBNA3B) is expressed in latently infected growth-transformed lymphocytes. J Virol. 1988 Jun;62(6):2173–2178. doi: 10.1128/jvi.62.6.2173-2178.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., Sample C., Kieff E. Subnuclear localization and phosphorylation of Epstein-Barr virus latent infection nuclear proteins. Virology. 1990 Jun;176(2):563–574. doi: 10.1016/0042-6822(90)90027-o. [DOI] [PubMed] [Google Scholar]
- Petti L., Sample J., Wang F., Kieff E. A fifth Epstein-Barr virus nuclear protein (EBNA3C) is expressed in latently infected growth-transformed lymphocytes. J Virol. 1988 Apr;62(4):1330–1338. doi: 10.1128/jvi.62.4.1330-1338.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack A., Delius H., Zimber U., Bornkamm G. W. Two deletions in the Epstein-Barr virus genome of the Burkitt lymphoma nonproducer line Raji. Virology. 1984 Feb;133(1):146–157. doi: 10.1016/0042-6822(84)90433-1. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Achong B. G., Epstein M. A. Cultivation and fine structure of virus-bearing lymphoblasts from a second New Guinea Burkitt lymphoma: establishment of sublines with unusual cultural properties. Int J Cancer. 1968 Mar 15;3(2):171–182. doi: 10.1002/ijc.2910030202. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N., Pritchett R., Kieff E. DNA of Epstein-Barr virus. III. Identification of restriction enzyme fragments that contain DNA sequences which differ among strains of Epstein-Barr virus. J Virol. 1978 Aug;27(2):388–398. doi: 10.1128/jvi.27.2.388-398.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M., Gradoville L., Heston L., Miller G. Non-immortalizing P3J-HR-1 Epstein-Barr virus: a deletion mutant of its transforming parent, Jijoye. J Virol. 1982 Dec;44(3):834–844. doi: 10.1128/jvi.44.3.834-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. B., Young L. S., Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J Virol. 1987 May;61(5):1310–1317. doi: 10.1128/jvi.61.5.1310-1317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricksten A., Kallin B., Alexander H., Dillner J., Fåhraeus R., Klein G., Lerner R., Rymo L. BamHI E region of the Epstein-Barr virus genome encodes three transformation-associated nuclear proteins. Proc Natl Acad Sci U S A. 1988 Feb;85(4):995–999. doi: 10.1073/pnas.85.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Cadwallader K., Petti L., Kieff E., Rickinson A. B. Distinction between Epstein-Barr virus type A (EBNA 2A) and type B (EBNA 2B) isolates extends to the EBNA 3 family of nuclear proteins. J Virol. 1989 Mar;63(3):1031–1039. doi: 10.1128/jvi.63.3.1031-1039.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Crocker J., Stokes H., Henderson S., Rickinson A. B. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991 Jan 1;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Young L., Martin B., Chatman T., Kieff E., Rickinson A., Kieff E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J Virol. 1990 Sep;64(9):4084–4092. doi: 10.1128/jvi.64.9.4084-4092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Yamaki M., Sakuma S., Ono Y., Takada K. Three Epstein-Barr virus (EBV)-determined nuclear antigens induced by the BamHI E region of EBV DNA. Int J Cancer. 1988 May 15;41(5):744–751. doi: 10.1002/ijc.2910410518. [DOI] [PubMed] [Google Scholar]
- Shope T., Dechairo D., Miller G. Malignant lymphoma in cottontop marmosets after inoculation with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2487–2491. doi: 10.1073/pnas.70.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixbey J. W., Vesterinen E. H., Nedrud J. G., Raab-Traub N., Walton L. A., Pagano J. S. Replication of Epstein-Barr virus in human epithelial cells infected in vitro. Nature. 1983 Dec 1;306(5942):480–483. doi: 10.1038/306480a0. [DOI] [PubMed] [Google Scholar]
- Skare J., Farley J., Strominger J. L., Fresen K. O., Cho M. S., zur Hausen H. Transformation by Epstein-Barr virus requires DNA sequences in the region of BamHI fragments Y and H. J Virol. 1985 Aug;55(2):286–297. doi: 10.1128/jvi.55.2.286-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal S. P., Ambinder R., Beschorner W. E., Hayward G. S., Mann R. A survey of Epstein-Barr virus DNA in lymphoid tissue. Frequent detection in Hodgkin's disease. Am J Clin Pathol. 1989 Jan;91(1):1–5. doi: 10.1093/ajcp/91.1.1. [DOI] [PubMed] [Google Scholar]
- Swaminathan S., Tomkinson B., Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1546–1550. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson B., Kieff E. Second-site homologous recombination in Epstein-Barr virus: insertion of type 1 EBNA 3 genes in place of type 2 has no effect on in vitro infection. J Virol. 1992 Feb;66(2):780–789. doi: 10.1128/jvi.66.2.780-789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson B., Kieff E. Use of second-site homologous recombination to demonstrate that Epstein-Barr virus nuclear protein 3B is not important for lymphocyte infection or growth transformation in vitro. J Virol. 1992 May;66(5):2893–2903. doi: 10.1128/jvi.66.5.2893-2903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbett B. E., Picchio G., Mosier D. E. hu-PBL-SCID mice: a model for human immune function, AIDS, and lymphomagenesis. Immunol Rev. 1991 Dec;124:139–164. doi: 10.1111/j.1600-065x.1991.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Walker L., Guy G., Brown G., Rowe M., Milner A. E., Gordon J. Control of human B-lymphocyte replication. I. Characterization of novel activation states that precede the entry of G0 B cells into cycle. Immunology. 1986 Aug;58(4):583–589. [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L., Alfieri C., Hennessy K., Evans H., O'Hara C., Anderson K. C., Ritz J., Shapiro R. S., Rickinson A., Kieff E. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989 Oct 19;321(16):1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970 Dec 12;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]