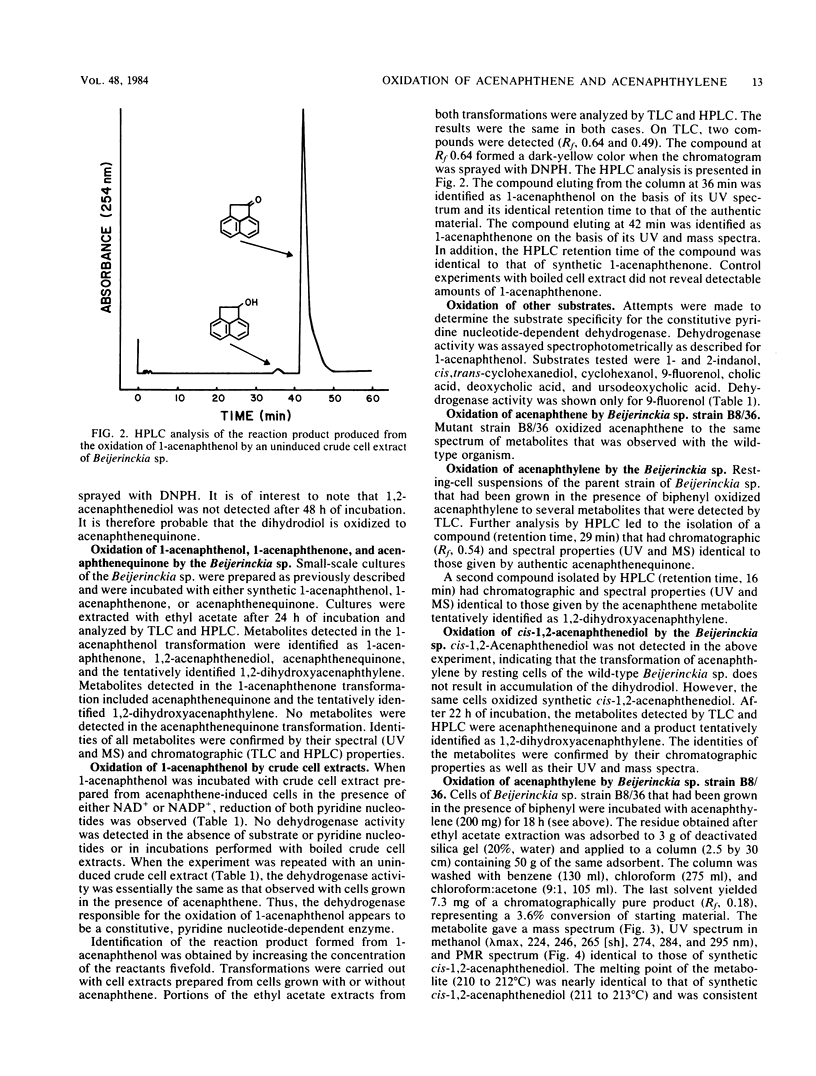
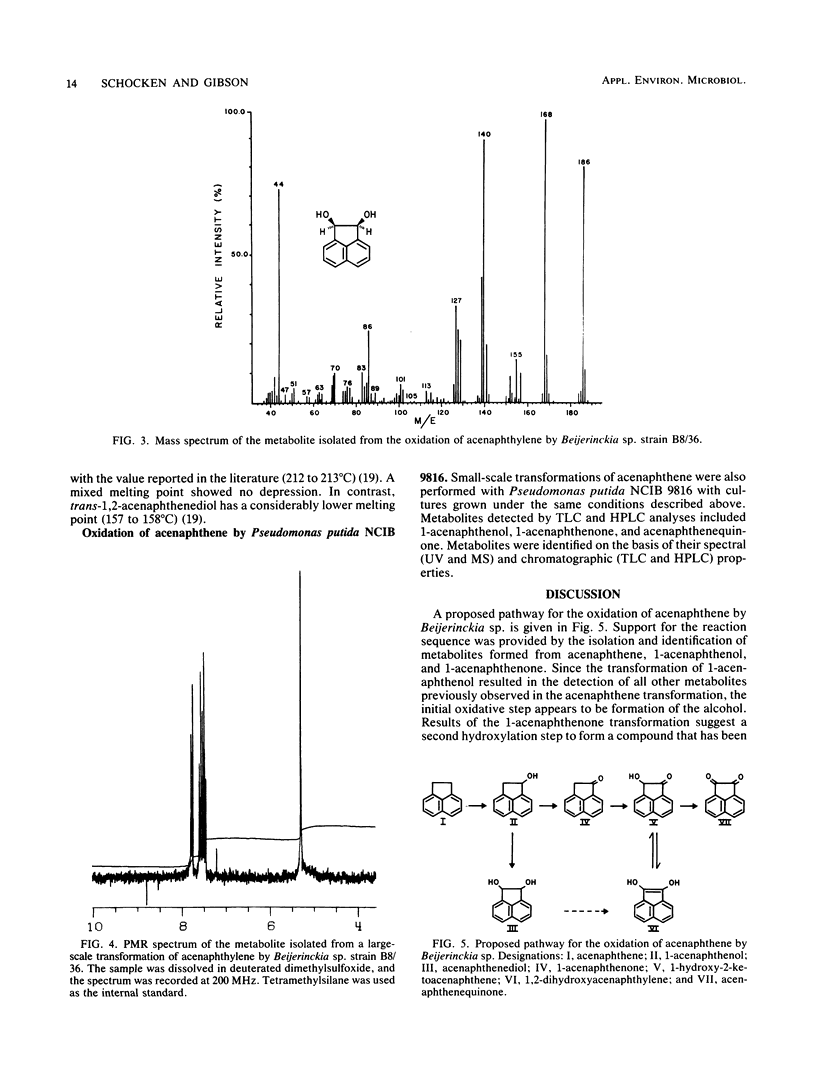
Abstract
A Beijerinckia sp. and a mutant strain, Beijerinckia sp. strain B8/36, were shown to cooxidize the polycyclic aromatic hydrocarbons acenaphthene and acenaphthylene. Both organisms oxidized acenaphthene to the same spectrum of metabolites, which included 1-acenaphthenol, 1-acenaphthenone, 1,2-acenaphthenediol, acenaphthenequinone, and a compound that was tentatively identified as 1,2-dihydroxyacenaphthylene. In contrast, acenaphthylene was oxidized to acenaphthenequinone and the compound tentatively identified as 1,2-dihydroxyacenaphthylene by the wild-type strain of Beijerinckia. Both of these products were also formed when the organism was incubated with synthetic cis-1,2-acenaphthenediol. A metabolite identified as cis-1,2-acenaphthenediol was formed from acenaphthylene by the mutant Beijerinckia sp. strain B8/36. Cell extracts prepared from the wild-type Beijerinckia strain contain a constitutive pyridine nucleotide-dependent dehydrogenase which can oxidize 1-acenaphthenol and 9-fluorenol. The results indicate that although acenaphthene and acenaphthylene are both oxidized to acenaphthenequinone, the pathways leading to the formation of this end product are different.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billings R. E., Sullivan H. R., McMahon R. E. The dehydrogenation of 1-indanol by a soluble oxidoreductase from bovine liver. J Biol Chem. 1971 Jun 10;246(11):3512–3517. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Morgan J. C., Gibson D. T. Bacterial and fungal oxidation of dibenzofuran. Biochem J. 1979 Apr 15;180(1):175–185. doi: 10.1042/bj1800175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond E. C., Callaghan P., Hopkins R. P. Metabolic dehydrogenation of cis- and trans-acenaphthene-1,2-diol. Xenobiotica. 1972 Nov;2(6):529–538. doi: 10.3109/00498257209111081. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Mahadevan V., Jerina D. M., Yogi H., Yeh H. J. Oxidation of the carcinogens benzo [a] pyrene and benzo [a] anthracene to dihydrodiols by a bacterium. Science. 1975 Jul 25;189(4199):295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- HOPKINS R. P., BROOKS C. J., YOUNG L. Biochemical studies of toxic agents. 13. The metabolism of acenaphthylene. Biochem J. 1962 Mar;82:457–466. doi: 10.1042/bj0820457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S., Masuda N. Characterization of NADP-dependent 7 beta-hydroxysteroid dehydrogenases from Peptostreptococcus productus and Eubacterium aerofaciens. Appl Environ Microbiol. 1982 May;43(5):1057–1063. doi: 10.1128/aem.43.5.1057-1063.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R. P. Microsomal ring-fission of cis- and trans-acenaphthene-1,2-diol. Biochem J. 1968 Jul;108(4):577–582. doi: 10.1042/bj1080577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R. P., Young L. Biochemical studies of toxic agents. Metabolic ring-fission of cis- and trans-acenaphthene-1,2-diol. Biochem J. 1966 Jan;98(1):19–24. doi: 10.1042/bj0980019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Sherrod J. A. Multiple forms of 7-alpha-hydroxysteroid dehydrogenase in selected strains of Bacteroides fragilis. J Bacteriol. 1975 May;122(2):418–424. doi: 10.1128/jb.122.2.418-424.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerina D. M., Selander H., Yagi H., Wells M. C., Davey J. F., Mahadevan V., Gibson D. T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976 Sep 15;98(19):5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Metabolism of Dibenzo-p-Dioxin and Chlorinated Dibenzo-p- Dioxins by a Beijerinckia Species. Appl Environ Microbiol. 1980 Feb;39(2):288–296. doi: 10.1128/aem.39.2.288-296.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde A. L., Gibson D. T. Metabolism of dibenzothiophene by a Beijerinckia species. Appl Environ Microbiol. 1977 Dec;34(6):783–790. doi: 10.1128/aem.34.6.783-790.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C. J., Hopkins R. P., Callaghan P. Metabolic oxidation of acenaphthen-1-ol. Xenobiotica. 1973 Oct;3(10):633–642. doi: 10.3109/00498257309151587. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Vogel K., Bentley P., Platt K. L., Oesch F. Rat liver cytoplasmic dihydrodiol dehydrogenase. Purification to apparent homogeneity and properties. J Biol Chem. 1980 Oct 25;255(20):9621–9625. [PubMed] [Google Scholar]


