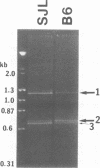
Abstract
The SJL mouse strain is resistant to infection by some strains of the murine coronavirus mouse hepatitis virus (MHV), such as JHM and A59. The block to virus infection has been variously attributed to defects in virus receptors or virus spread. Since the cellular receptors for MHV, mmCGM1 and mmCGM2, have recently been identified as members of the carcinoembryonic antigen family, we reexamined the possible defectiveness of the MHV receptors in SJL mouse strain. Cloning and sequencing of the cDNAs of both mmCGMs RNAs from SJL mice revealed that they were identical in size to those of the susceptible C57BL/6 (B6) mouse. There was some sequence divergence in the N terminus of the mmCGM molecules between the two mouse strains, resulting in a different number of potential glycosylation sites. This was confirmed by in vitro translation of the mmCGM RNAs, which showed that the glycosylated mmCGM2 of SJL was smaller than that of B6 mice. However, transfection of either mmCGM1 or mmCGM2 from SJL mice into MHV-resistant Cos 7 cells rendered the cells susceptible to MHV infection. The ability of the SJL mmCGM molecules to serve as MHV receptors was comparable to that of those from B6. These molecules are expressed in SJL mouse brain and liver in a similar ratio and in amounts equivalent to those in the B6 mouse. Furthermore, we demonstrated that an SJL-derived cell line was susceptible to A59 but resistant to JHM infection. We concluded that the MHV receptor molecules in the SJL mouse are functional and that the resistance of SJL mice to infection by some MHV strains most likely results from some other factor(s) required for virus entry or some other step(s) in virus replication.
Full text
PDF

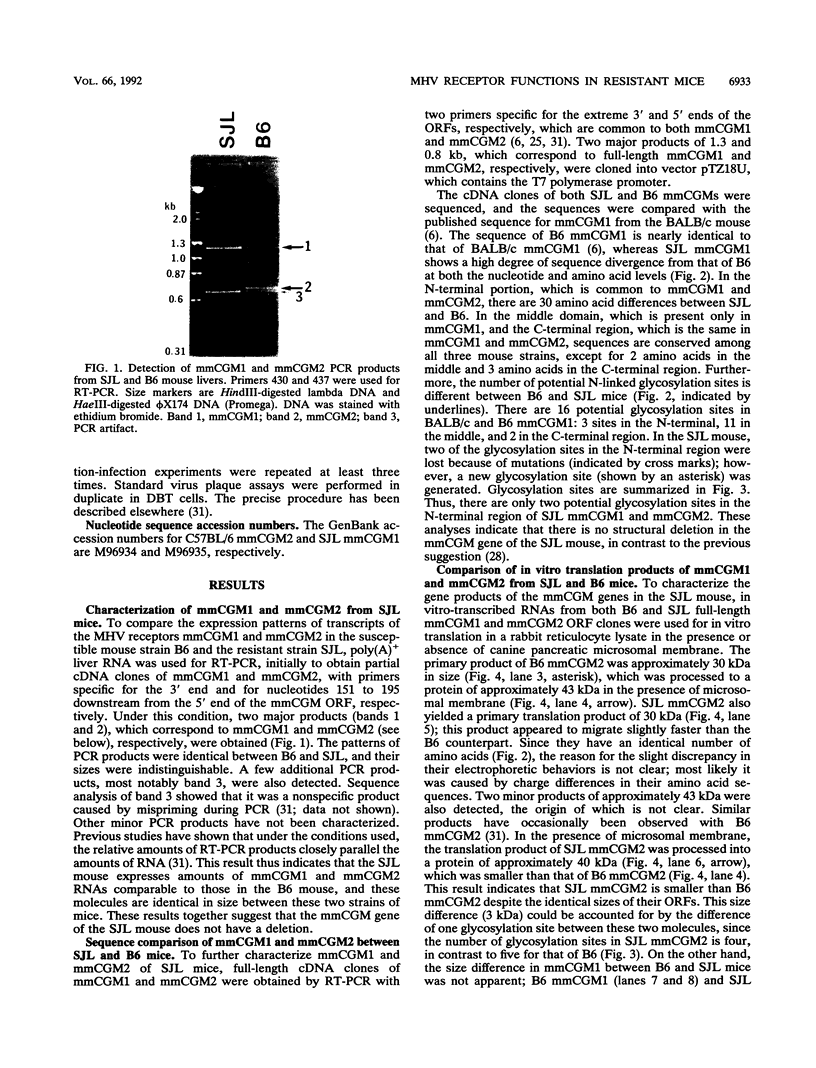

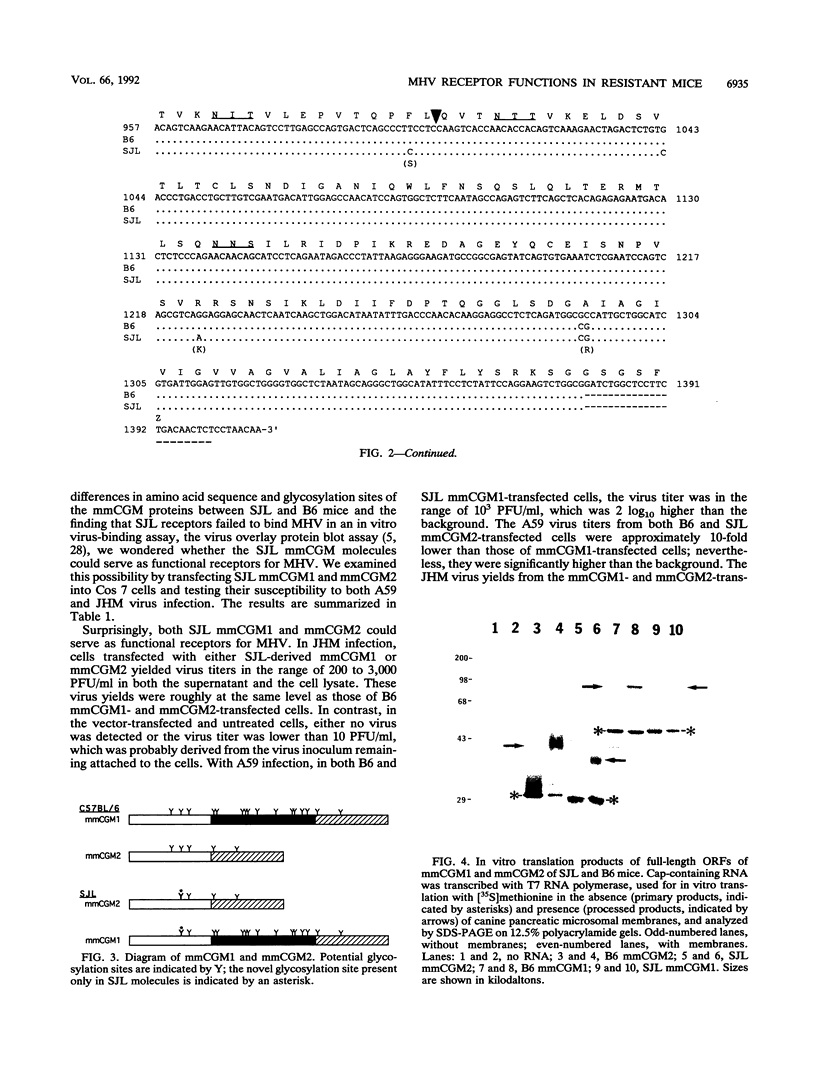



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang F. B. The use of a genetically incompatible combination of host and virus (MHV) for the study of mechanisms of host resistance. Adv Exp Med Biol. 1981;142:359–373. doi: 10.1007/978-1-4757-0456-3_30. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Beck D. S., Smith A. L. Mouse hepatitis virus nasoencephalopathy is dependent upon virus strain and host genotype. Arch Virol. 1986;91(3-4):247–256. doi: 10.1007/BF01314284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W. Host age and genotypic effects on enterotropic mouse hepatitis virus infection. Lab Anim Sci. 1987 Feb;37(1):36–40. [PubMed] [Google Scholar]
- Barthold S. W., Smith A. L. Response of genetically susceptible and resistant mice to intranasal inoculation with mouse hepatitis virus JHM. Virus Res. 1987 May;7(3):225–239. doi: 10.1016/0168-1702(87)90030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Weismiller D. G., Holmes K. V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987 Jan;61(1):185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dveksler G. S., Pensiero M. N., Cardellichio C. B., Williams R. K., Jiang G. S., Holmes K. V., Dieffenbach C. W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991 Dec;65(12):6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Van Dinter S. Several rat cell lines share a common defect in their inability to internalize murine coronaviruses efficiently. J Gen Virol. 1989 Jul;70(Pt 7):1713–1724. doi: 10.1099/0022-1317-70-7-1713. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Hirano N., Fujiwara K., Hino S., Matumoto M. Replication and plaque formation of mouse hepatitis virus (MHV-2) in mouse cell line DBT culture. Arch Gesamte Virusforsch. 1974;44(3):298–302. doi: 10.1007/BF01240618. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Haspel M. V., Oldstone M. B. Mouse hepatitis virus type 4 (JHM strains). induced fatal central nervous system disease. I. genetic control and murine neuron as the susceptible site of disease. J Exp Med. 1981 Apr 1;153(4):832–843. doi: 10.1084/jem.153.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler R. L., Taylor B. A., Wooddell M. K., Beamer W. G., Oldstone M. B. Host genetic control of mouse hepatitis virus type-4 (JHM strain) replication. II. The gene locus for susceptibility is linked to the Svp-2 locus on mouse chromosome 7. Exp Clin Immunogenet. 1984;1(4):217–222. [PubMed] [Google Scholar]
- Knobler R. L., Tunison L. A., Lampert P. W., Oldstone M. B. Selected mutants of mouse hepatitis virus type 4 (JHM strain) induce different CNS diseases. Pathobiology of disease induced by wild type and mutants ts8 and ts15 in BALB/c and SJL/J mice. Am J Pathol. 1982 Nov;109(2):157–168. [PMC free article] [PubMed] [Google Scholar]
- Knobler R. L., Tunison L. A., Oldstone M. B. Host genetic control of mouse hepatitis virus type 4 (JHM strain) replication. I. Restriction of virus amplification and spread in macrophages from resistant mice. J Gen Virol. 1984 Sep;65(Pt 9):1543–1548. doi: 10.1099/0022-1317-65-9-1543. [DOI] [PubMed] [Google Scholar]
- Macnaughton T. B., Gowans E. J., Jilbert A. R., Burrell C. J. Hepatitis delta virus RNA, protein synthesis and associated cytotoxicity in a stably transfected cell line. Virology. 1990 Aug;177(2):692–698. doi: 10.1016/0042-6822(90)90535-y. [DOI] [PubMed] [Google Scholar]
- Makino S., Lai M. M. Evolution of the 5'-end of genomic RNA of murine coronaviruses during passages in vitro. Virology. 1989 Mar;169(1):227–232. doi: 10.1016/0042-6822(89)90060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Taguchi F., Hirano N., Fujiwara K. Analysis of genomic and intracellular viral RNAs of small plaque mutants of mouse hepatitis virus, JHM strain. Virology. 1984 Nov;139(1):138–151. doi: 10.1016/0042-6822(84)90335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensiero M. N., Dveksler G. S., Cardellichio C. B., Jiang G. S., Elia P. E., Dieffenbach C. W., Holmes K. V. Binding of the coronavirus mouse hepatitis virus A59 to its receptor expressed from a recombinant vaccinia virus depends on posttranslational processing of the receptor glycoprotein. J Virol. 1992 Jul;66(7):4028–4039. doi: 10.1128/jvi.66.7.4028-4039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Bond C. W. Pathogenic murine coronaviruses. I. Characterization of biological behavior in vitro and virus-specific intracellular RNA of strongly neurotropic JHMV and weakly neurotropic A59V viruses. Virology. 1979 Apr 30;94(2):352–370. doi: 10.1016/0042-6822(79)90467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh C. K., Lee H. J., Yokomori K., La Monica N., Makino S., Lai M. M. Identification of a new transcriptional initiation site and the corresponding functional gene 2b in the murine coronavirus RNA genome. J Virol. 1989 Sep;63(9):3729–3736. doi: 10.1128/jvi.63.9.3729-3736.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Winograd D. F., de Souza M. S. In vitro splenic T cell responses of diverse mouse genotypes after oronasal exposure to mouse hepatitis virus, strain JHM. Lab Anim Sci. 1991 Apr;41(2):106–111. [PubMed] [Google Scholar]
- Stohlman S. A., Frelinger J. A., Weiner L. P. Resistance to fatal central nervous system disease by mouse hepatitis virus, strain JHM. II. Adherent cell-mediated protection. J Immunol. 1980 Apr;124(4):1733–1739. [PubMed] [Google Scholar]
- Tardieu M., Goffinet A., Harmant-van Rijckevorsel G., Lyon G. Ependymitis, leukoencephalitis, hydrocephalus, and thrombotic vasculitis following chronic infection by mouse hepatitis virus 3 (MHV 3). Acta Neuropathol. 1982;58(3):168–176. doi: 10.1007/BF00690797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbide C., Rojas M., Stanners C. P., Beauchemin N. A mouse carcinoembryonic antigen gene family member is a calcium-dependent cell adhesion molecule. J Biol Chem. 1991 Jan 5;266(1):309–315. [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Holmes K. V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Snyder S. W., Frana M. F., Holmes K. V. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J Virol. 1990 Aug;64(8):3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Dales S. In vivo and in vitro models of demyelinating disease: efficiency of virus spread and formation of infectious centers among glial cells is genetically determined by the murine host. J Virol. 1988 Sep;62(9):3371–3377. doi: 10.1128/jvi.62.9.3371-3377.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori K., Banner L. R., Lai M. M. Heterogeneity of gene expression of the hemagglutinin-esterase (HE) protein of murine coronaviruses. Virology. 1991 Aug;183(2):647–657. doi: 10.1016/0042-6822(91)90994-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori K., La Monica N., Makino S., Shieh C. K., Lai M. M. Biosynthesis, structure, and biological activities of envelope protein gp65 of murine coronavirus. Virology. 1989 Dec;173(2):683–691. doi: 10.1016/0042-6822(89)90581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori K., Lai M. M. Mouse hepatitis virus utilizes two carcinoembryonic antigens as alternative receptors. J Virol. 1992 Oct;66(10):6194–6199. doi: 10.1128/jvi.66.10.6194-6199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]