Abstract
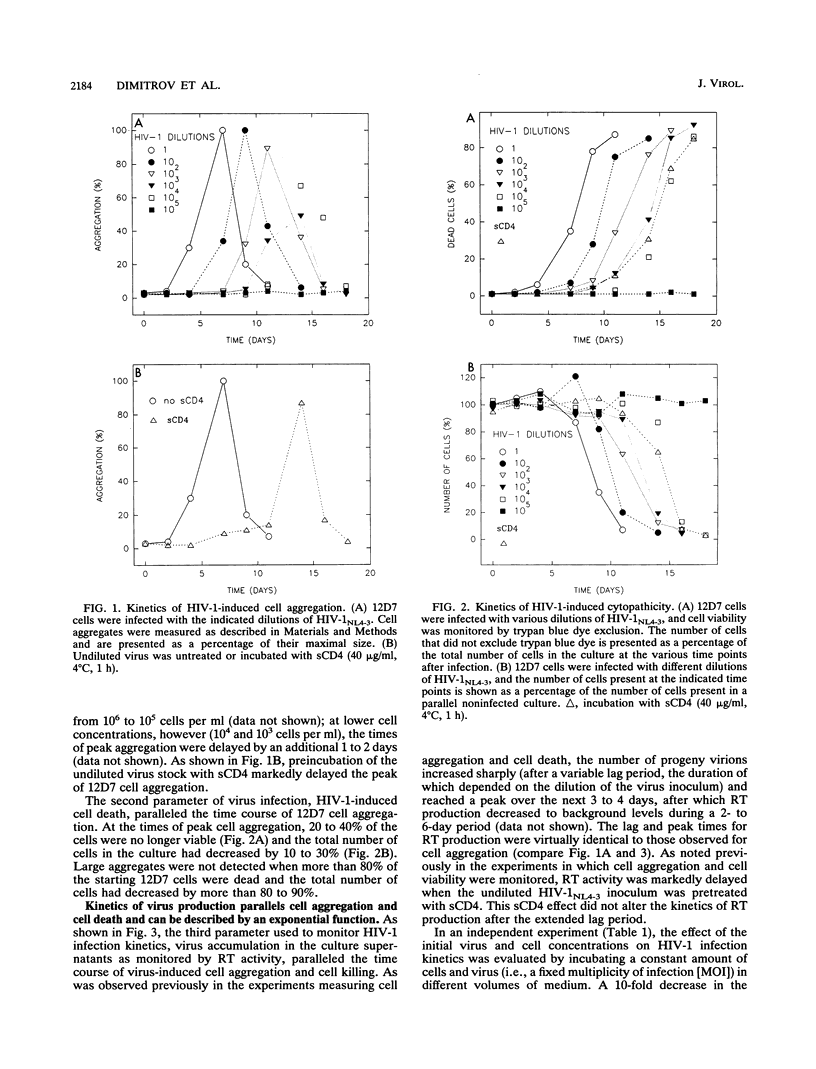
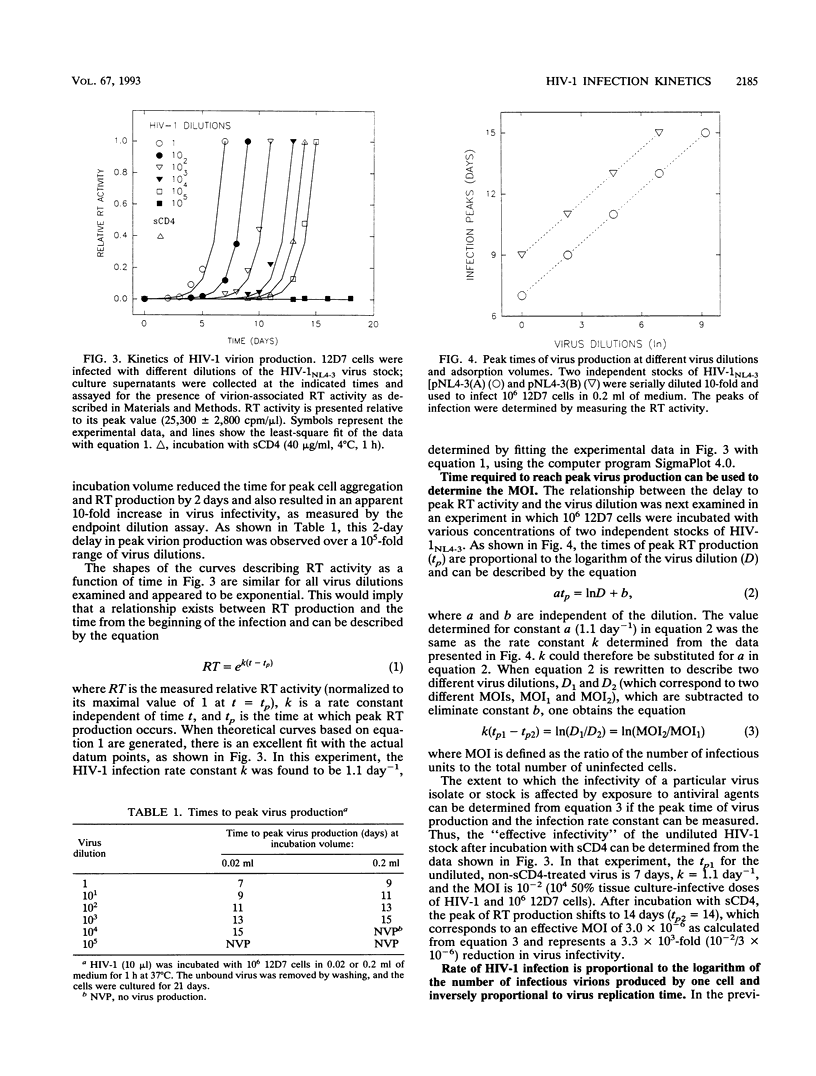
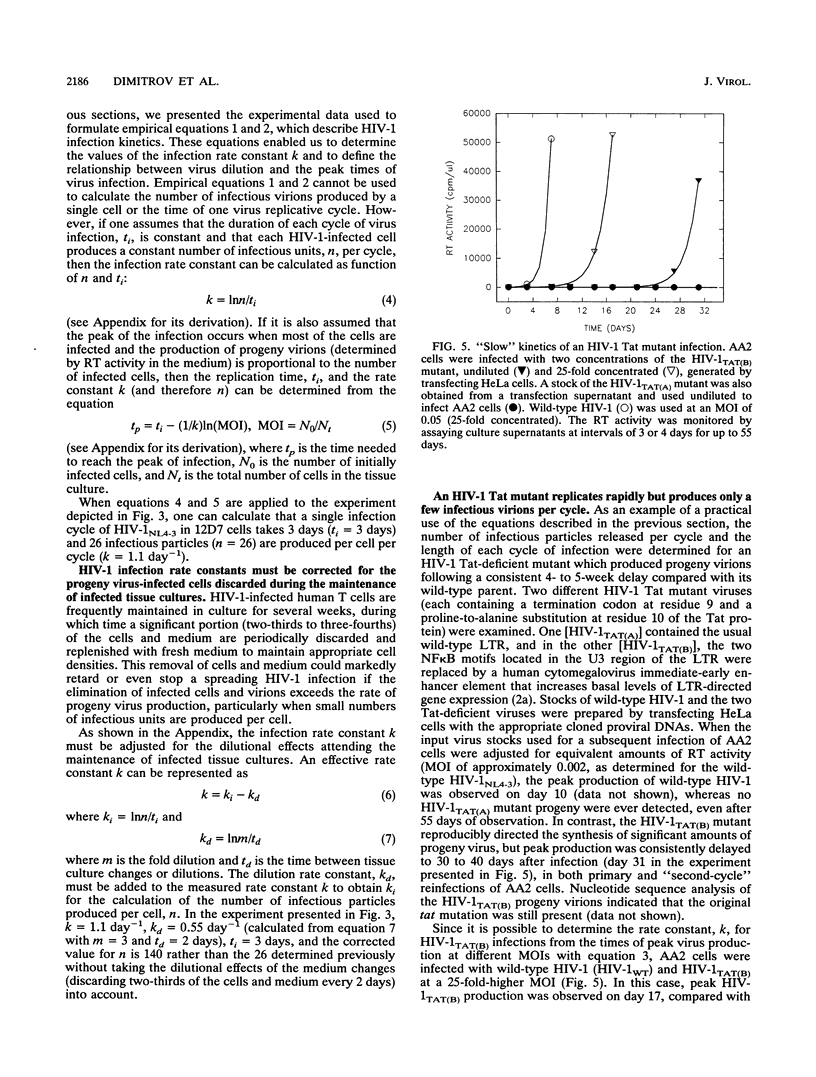
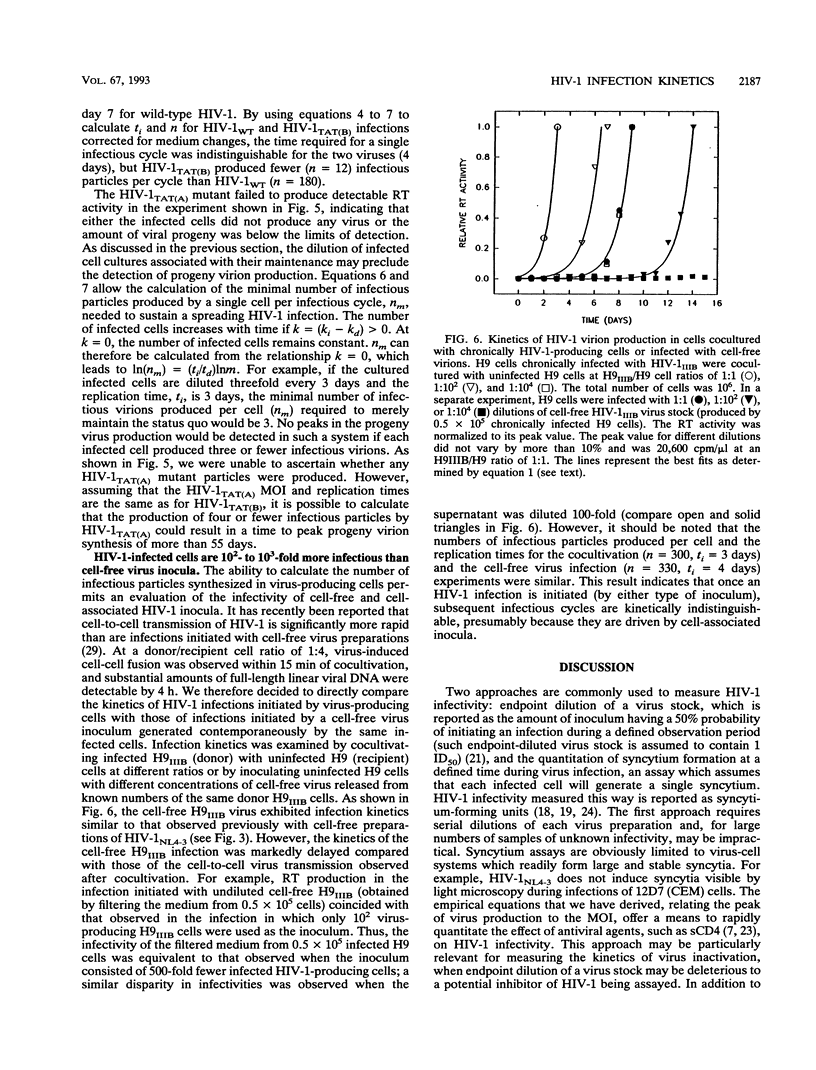
Tissue culture infections of CD4-positive human T cells by human immunodeficiency virus type 1 (HIV-1) proceed in three stages: (i) a period following the initiation of an infection during which no detectable virus is produced; (ii) a phase in which a sharp increase followed by a peak of released progeny virions can be measured; and (iii) a final period when virus production declines. In this study, we have derived equations describing the kinetics of HIV-1 accumulation in cell culture supernatants during multiple rounds of infection. Our analyses indicated that the critical parameter affecting the kinetics of HIV-1 infection is the infection rate constant k = Inn/ti, where n is the number of infectious virions produced by one cell (about 10(2)) and ti is the time required for one complete cycle of virus infection (typically 3 to 4 days). Of particular note was our finding that the infectivity of HIV-1 during cell-to-cell transmission is 10(2) to 10(3) times greater than the infectivity of cell-free virus stocks, the inocula commonly used to initiate tissue culture infections. We also demonstrated that the slow infection kinetics of an HIV-1 tat mutant is not due to a longer replication time but reflects the small number of infectious particles produced per cycle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee S., Leeds J. M., Matthews T. J., Weinhold K. J., Skinner M., Bolognesi D. P., Hershfield M. S. Phenotypic variation in the response to the human immunodeficiency virus among derivatives of the CEM T and WIL-2 B cell lines. J Exp Med. 1988 Aug 1;168(2):605–621. doi: 10.1084/jem.168.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Shioda T., Levy J. A. Host range, replicative, and cytopathic properties of human immunodeficiency virus type 1 are determined by very few amino acid changes in tat and gp120. J Virol. 1991 Dec;65(12):6931–6941. doi: 10.1128/jvi.65.12.6931-6941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. J., Saag M. S., Decker W. D., Campbell-Hill S., Roberson J. L., Veldkamp P. J., Kappes J. C., Hahn B. H., Shaw G. M. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991 Apr 4;324(14):954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Moudgil T., Meyer R. D., Ho D. D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Apr 4;324(14):961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Golding H., Blumenthal R. Initial stages of HIV-1 envelope glycoprotein-mediated cell fusion monitored by a new assay based on redistribution of fluorescent dyes. AIDS Res Hum Retroviruses. 1991 Oct;7(10):799–805. doi: 10.1089/aid.1991.7.799. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Willey R. L., Martin M. A., Blumenthal R. Kinetics of HIV-1 interactions with sCD4 and CD4+ cells: implications for inhibition of virus infection and initial steps of virus entry into cells. Virology. 1992 Apr;187(2):398–406. doi: 10.1016/0042-6822(92)90441-q. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Albert J., Asjö B. Replicative capacity, cytopathic effect and cell tropism of HIV. AIDS. 1989;3 (Suppl 1):S5–12. doi: 10.1097/00002030-198901001-00002. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Morfeldt-Månson L., Chiodi F., Lind B., von Gegerfelt A., Albert J., Olausson E., Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988 Nov;62(11):4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines H., Albert J., von Sydow M., Sönnerborg A., Chiodi F., Ehrnst A., Strannegård O., Asjö B. HIV antigenaemia and virus isolation from plasma during primary HIV infection. Lancet. 1987 Jun 6;1(8545):1317–1318. doi: 10.1016/s0140-6736(87)90570-8. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Sarngadharan M. G., Resnick L., Dimarzoveronese F., Rota T. R., Hirsch M. S. Primary human T-lymphotropic virus type III infection. Ann Intern Med. 1985 Dec;103(6 ):880–883. doi: 10.7326/0003-4819-103-6-880. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Monick M. M., Liu B., Stinski M. F. The promoter-regulatory region of the major immediate-early gene of human cytomegalovirus responds to T-lymphocyte stimulation and contains functional cyclic AMP-response elements. J Virol. 1989 Jul;63(7):3026–3033. doi: 10.1128/jvi.63.7.3026-3033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991 May 17;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kiernan R., Marshall J., Bowers R., Doherty R., McPhee D. Kinetics of HIV-1 replication and intracellular accumulation of particles in HTLV-I-transformed cells. AIDS Res Hum Retroviruses. 1990 Jun;6(6):743–752. doi: 10.1089/aid.1990.6.743. [DOI] [PubMed] [Google Scholar]
- Layne S. P., Merges M. J., Dembo M., Spouge J. L., Conley S. R., Moore J. P., Raina J. L., Renz H., Gelderblom H. R., Nara P. L. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992 Aug;189(2):695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- Layne S. P., Merges M. J., Dembo M., Spouge J. L., Nara P. L. HIV requires multiple gp120 molecules for CD4-mediated infection. Nature. 1990 Jul 19;346(6281):277–279. doi: 10.1038/346277a0. [DOI] [PubMed] [Google Scholar]
- Layne S. P., Merges M. J., Spouge J. L., Dembo M., Nara P. L. Blocking of human immunodeficiency virus infection depends on cell density and viral stock age. J Virol. 1991 Jun;65(6):3293–3300. doi: 10.1128/jvi.65.6.3293-3300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy R. J., Sarkar D. P., Chen Y., Blumenthal R. Observation of single influenza virus-cell fusion and measurement by fluorescence video microscopy. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1850–1854. doi: 10.1073/pnas.87.5.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Cort S. P., Kennedy M. S., Cabridilla C. D., Feorino P. M., Francis D. P., Hicks D., Kalyanaraman V. S., Martin L. S. Immunoassay for the detection and quantitation of infectious human retrovirus, lymphadenopathy-associated virus (LAV). J Immunol Methods. 1985 Jan 21;76(1):171–183. doi: 10.1016/0022-1759(85)90489-2. [DOI] [PubMed] [Google Scholar]
- McKeating J. A., McKnight A., McIntosh K., Clapham P. R., Mulder C., Weiss R. A. Evaluation of human and simian immunodeficiency virus plaque and neutralization assays. J Gen Virol. 1989 Dec;70(Pt 12):3327–3333. doi: 10.1099/0022-1317-70-12-3327. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Fischinger P. J. Quantitative infectivity assay for HIV-1 and-2. Nature. 1988 Mar 31;332(6163):469–470. doi: 10.1038/332469a0. [DOI] [PubMed] [Google Scholar]
- Nowak M. A., Anderson R. M., McLean A. R., Wolfs T. F., Goudsmit J., May R. M. Antigenic diversity thresholds and the development of AIDS. Science. 1991 Nov 15;254(5034):963–969. doi: 10.1126/science.1683006. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. G., Li G., Potash M. J., Volsky D. J. Contribution of multiple rounds of viral entry and reverse transcription to expression of human immunodeficiency virus type 1. A quantitative kinetic study. J Biol Chem. 1991 Jan 25;266(3):1783–1788. [PubMed] [Google Scholar]
- Sato H., Orenstein J., Dimitrov D., Martin M. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology. 1992 Feb;186(2):712–724. doi: 10.1016/0042-6822(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Tersmette M., de Goede R. E., Al B. J., Winkel I. N., Gruters R. A., Cuypers H. T., Huisman H. G., Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988 Jun;62(6):2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volsky D. J., Pellegrino M. G., Li G., Logan K. A., Aswell J. E., Lawrence N. P., Decker S. R. Titration of human immunodeficiency virus type 1 (HIV-1) and quantitative analysis of virus expression in vitro using liquid RNA-RNA hybridization. J Virol Methods. 1990 Jun;28(3):257–271. doi: 10.1016/0166-0934(90)90119-z. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


