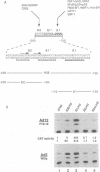
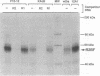
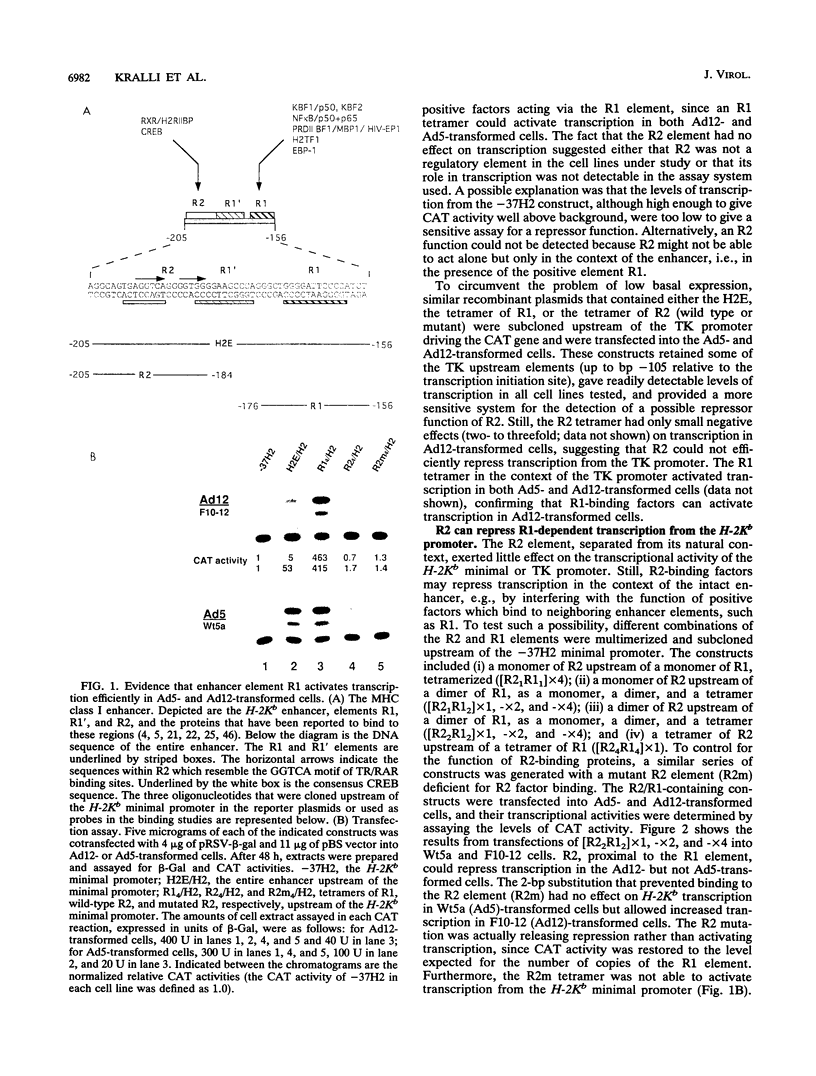
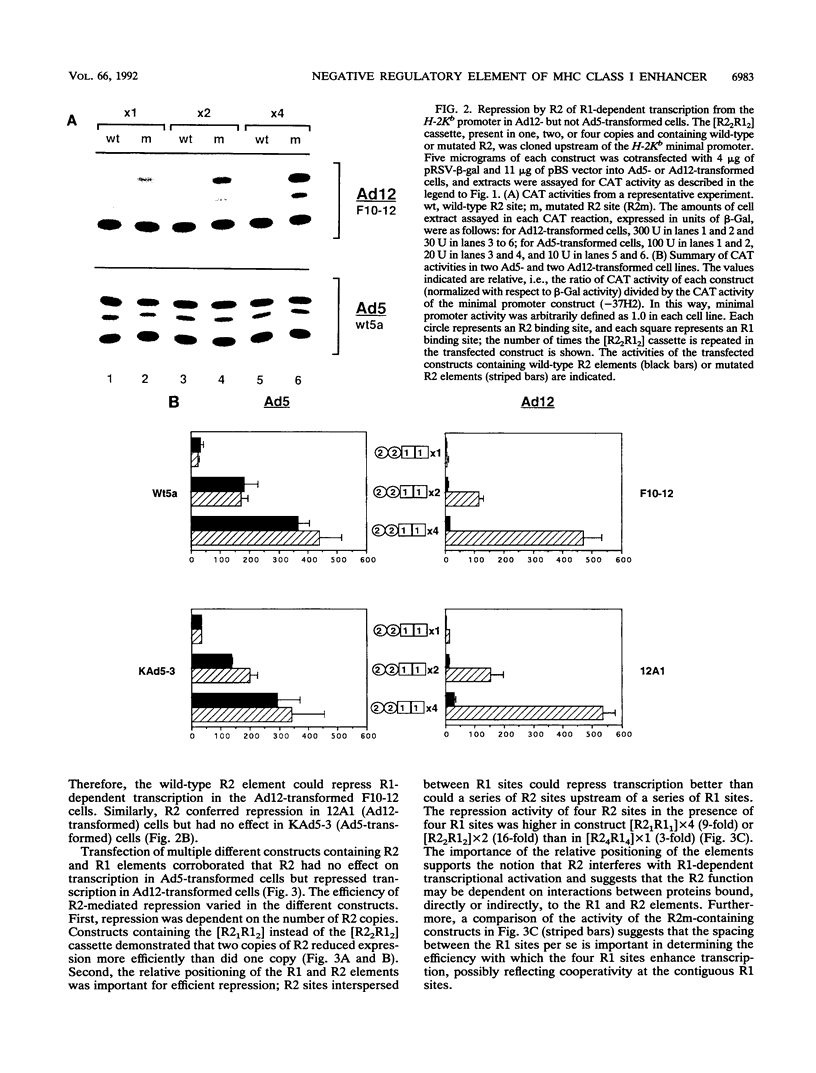
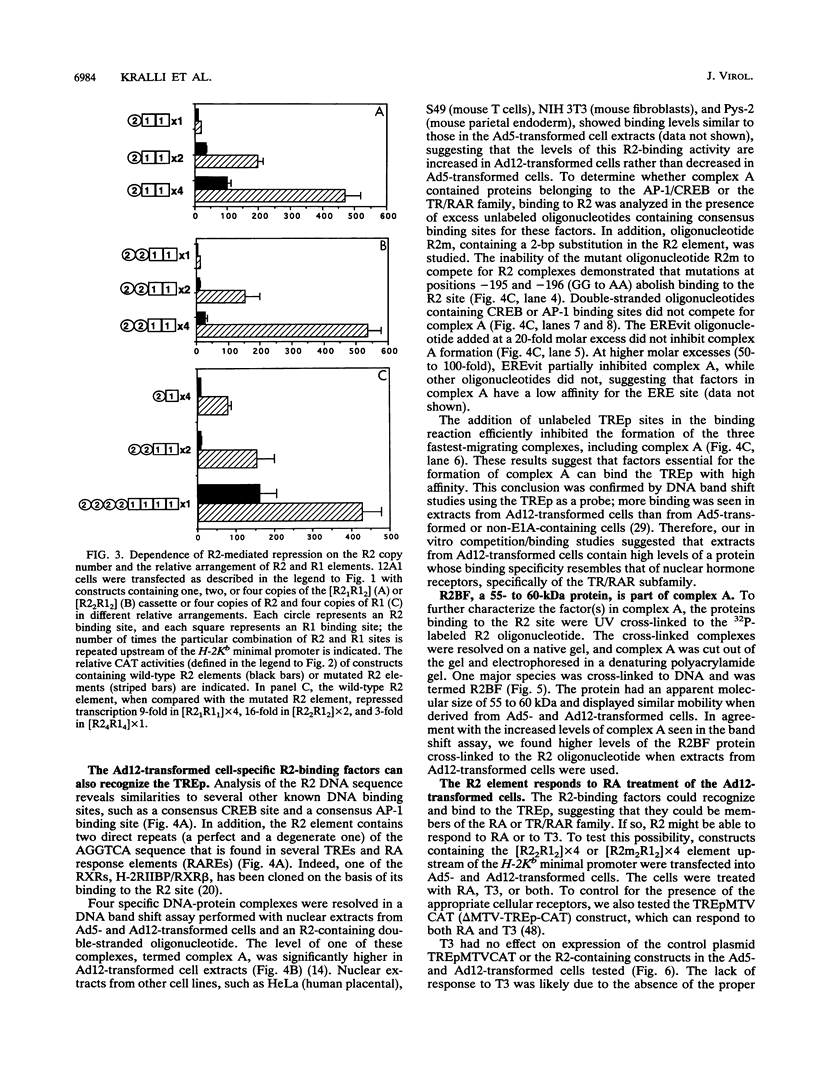
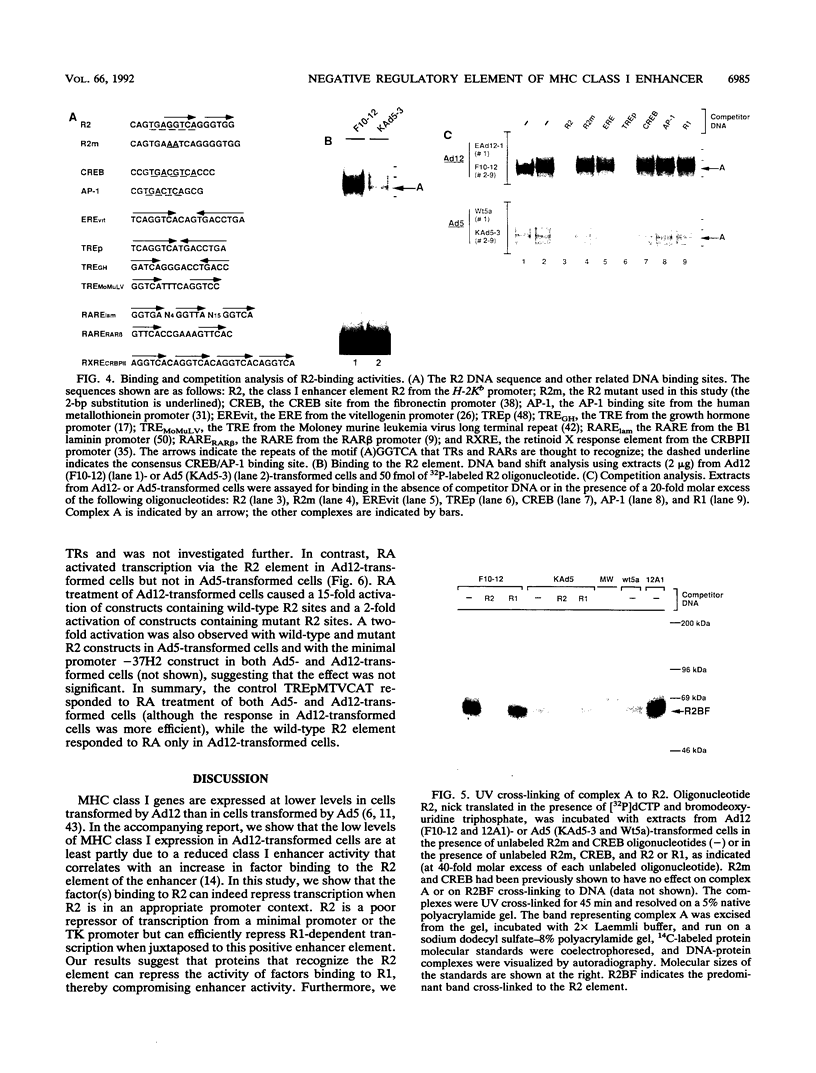
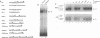Abstract
In cells transformed by the highly oncogenic adenovirus type 12 (Ad12), the viral E1A proteins mediate transcriptional repression of the major histocompatibility class I genes. In contrast, class I transcription is not reduced in cells transformed by the nononcogenic Ad5. The decreased rate of class I transcription is, at least in part, the result of a reduced major histocompatibility complex class I enhancer activity in Ad12-transformed cells and correlates with an increase in the levels of a DNA-binding activity to the R2 element of the enhancer (R. Ge, A. Kralli, R. Weinmann, and R. P. Ricciardi, J. Virol. 66:6969-6978, 1992). Employing transient transfection assays, we now provide direct evidence that the R2 element can confer repression in Ad12- but not Ad5-transformed cells. Repression by R2 was observed only in the presence of the positive enhancer element R1 and was dependent on (i) the number of the R2 elements and (ii) the relative arrangement of R2 and R1 elements. The putative R2-binding repressor protein, R2BF, was similar in molecular weight and binding specificity to members of the thyroid hormone/retinoic acid (RA) receptor family. RA treatment abrogated the R2-mediated repression in Ad12-transformed cells and had no effect on the activity of R2/R1-containing promoters in Ad5-transformed cells. These results are consistent with the presence of an R2-binding repressor in Ad12-transformed cells. In the absence of RA, the repressor compromises enhancer activity by interfering with the activity of the positive cis element R1. RA treatment of Ad12-transformed cells may render the repressor inactive.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrill A. M., Blair G. E. Nuclear proteins binding to an enhancer element of the major histocompatibility class I promoter: differences between highly oncogenic and nononcogenic adenovirus-transformed rat cells. Virology. 1989 Oct;172(2):643–646. doi: 10.1016/0042-6822(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Ackrill A. M., Blair G. E. Regulation of major histocompatibility class I gene expression at the level of transcription in highly oncogenic adenovirus transformed rat cells. Oncogene. 1988 Oct;3(4):483–487. [PubMed] [Google Scholar]
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, LeClair K. P., Singh H., Sharp P. A. A large protein containing zinc finger domains binds to related sequence elements in the enhancers of the class I major histocompatibility complex and kappa immunoglobulin genes. Mol Cell Biol. 1990 Apr;10(4):1406–1414. doi: 10.1128/mcb.10.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Binding of a nuclear factor to a regulatory sequence in the promoter of the mouse H-2Kb class I major histocompatibility gene. Mol Cell Biol. 1987 Jan;7(1):305–313. doi: 10.1128/mcb.7.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Burke P. A., Hirschfeld S., Shirayoshi Y., Kasik J. W., Hamada K., Appella E., Ozato K. Developmental and tissue-specific expression of nuclear proteins that bind the regulatory element of the major histocompatibility complex class I gene. J Exp Med. 1989 Apr 1;169(4):1309–1321. doi: 10.1084/jem.169.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Watine B., Israël A., Kourilsky P. The regulation and expression of MHC class I genes. Immunol Today. 1990 Aug;11(8):286–292. doi: 10.1016/0167-5699(90)90114-o. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eager K. B., Williams J., Breiding D., Pan S., Knowles B., Appella E., Ricciardi R. P. Expression of histocompatibility antigens H-2K, -D, and -L is reduced in adenovirus-12-transformed mouse cells and is restored by interferon gamma. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5525–5529. doi: 10.1073/pnas.82.16.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. M., Maniatis T. A DNA-binding protein containing two widely separated zinc finger motifs that recognize the same DNA sequence. Genes Dev. 1990 Jan;4(1):29–42. doi: 10.1101/gad.4.1.29. [DOI] [PubMed] [Google Scholar]
- Friedman D. J., Ricciardi R. P. Adenovirus type 12 E1A gene represses accumulation of MHC class I mRNAs at the level of transcription. Virology. 1988 Jul;165(1):303–305. doi: 10.1016/0042-6822(88)90689-7. [DOI] [PubMed] [Google Scholar]
- Ge R., Kralli A., Weinmann R., Ricciardi R. P. Down-regulation of the major histocompatibility complex class I enhancer in adenovirus type 12-transformed cells is accompanied by an increase in factor binding. J Virol. 1992 Dec;66(12):6969–6978. doi: 10.1128/jvi.66.12.6969-6978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Giguère V., Yang N., Segui P., Evans R. M. Identification of a new class of steroid hormone receptors. Nature. 1988 Jan 7;331(6151):91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Franco R., Weinberger C., Albert V. R., Evans R. M., Rosenfeld M. G. A c-erb-A binding site in rat growth hormone gene mediates trans-activation by thyroid hormone. Nature. 1987 Oct 22;329(6141):738–741. doi: 10.1038/329738a0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hamada K., Gleason S. L., Levi B. Z., Hirschfeld S., Appella E., Ozato K. H-2RIIBP, a member of the nuclear hormone receptor superfamily that binds to both the regulatory element of major histocompatibility class I genes and the estrogen response element. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8289–8293. doi: 10.1073/pnas.86.21.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël A., Kimura A., Kieran M., Yano O., Kanellopoulos J., Le Bail O., Kourilsky P. A common positive trans-acting factor binds to enhancer sequences in the promoters of mouse H-2 and beta 2-microglobulin genes. Proc Natl Acad Sci U S A. 1987 May;84(9):2653–2657. doi: 10.1073/pnas.84.9.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël A., Le Bail O., Hatat D., Piette J., Kieran M., Logeat F., Wallach D., Fellous M., Kourilsky P. TNF stimulates expression of mouse MHC class I genes by inducing an NF kappa B-like enhancer binding activity which displaces constitutive factors. EMBO J. 1989 Dec 1;8(12):3793–3800. doi: 10.1002/j.1460-2075.1989.tb08556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleher C. A., Goutte C., Johnson A. D. The yeast cell-type-specific repressor alpha 2 acts cooperatively with a non-cell-type-specific protein. Cell. 1988 Jun 17;53(6):927–936. doi: 10.1016/s0092-8674(88)90449-7. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Meijer I., Jochemsen A. G., de Wit C. M., Bos J. L., Morello D., van der Eb A. J. Adenovirus type 12 E1A down regulates expression of a transgene under control of a major histocompatibility complex class I promoter: evidence for transcriptional control. J Virol. 1989 Sep;63(9):4039–4042. doi: 10.1128/jvi.63.9.4039-4042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer I., van Dam H., Boot A. J., Bos J. L., Zantema A., van der Eb A. J. Co-regulated expression of junB and MHC class I genes in adenovirus-transformed cells. Oncogene. 1991 Jun;6(6):911–916. [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Nagata T., Segars J. H., Levi B. Z., Ozato K. Retinoic acid-dependent transactivation of major histocompatibility complex class I promoters by the nuclear hormone receptor H-2RIIBP in undifferentiated embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):937–941. doi: 10.1073/pnas.89.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsch U., Zimmer S. G., Babiss L. E. Changes in NF-kappa B and ISGF3 DNA binding activities are responsible for differences in MHC and beta-IFN gene expression in Ad5- versus Ad12-transformed cells. EMBO J. 1991 Dec;10(13):4169–4175. doi: 10.1002/j.1460-2075.1991.tb04995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Sap J., de Magistris L., Stunnenberg H., Vennström B. A major thyroid hormone response element in the third intron of the rat growth hormone gene. EMBO J. 1990 Mar;9(3):887–896. doi: 10.1002/j.1460-2075.1990.tb08186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Shirayoshi Y., Miyazaki J., Burke P. A., Hamada K., Appella E., Ozato K. Binding of multiple nuclear factors to the 5' upstream regulatory element of the murine major histocompatibility class I gene. Mol Cell Biol. 1987 Dec;7(12):4542–4548. doi: 10.1128/mcb.7.12.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Yoshioka T., Bieberich C., Jay G. Role of the major histocompatibility complex class I antigens in tumor growth and metastasis. Annu Rev Immunol. 1988;6:359–380. doi: 10.1146/annurev.iy.06.040188.002043. [DOI] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Vaessen R. T., Houweling A., Israel A., Kourilsky P., van der Eb A. J. Adenovirus E1A-mediated regulation of class I MHC expression. EMBO J. 1986 Feb;5(2):335–341. doi: 10.1002/j.1460-2075.1986.tb04217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasios G. W., Gold J. D., Petkovich M., Chambon P., Gudas L. J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Ing N. H., Tsai S. Y., O'Malley B. W., Tsai M. J. The COUP-TFs compose a family of functionally related transcription factors. Gene Expr. 1991;1(3):207–216. [PMC free article] [PubMed] [Google Scholar]
- Yano O., Kanellopoulos J., Kieran M., Le Bail O., Israël A., Kourilsky P. Purification of KBF1, a common factor binding to both H-2 and beta 2-microglobulin enhancers. EMBO J. 1987 Nov;6(11):3317–3324. doi: 10.1002/j.1460-2075.1987.tb02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Lehmann J., Hoffmann B., Dawson M. I., Cameron J., Graupner G., Hermann T., Tran P., Pfahl M. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992 Aug 13;358(6387):587–591. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]