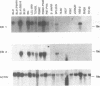
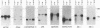Abstract
Since Epstein-Barr virus (EBV) infection of Burkitt's lymphoma (BL) cells in vitro reproduces many of the activation effects of EBV infection of primary B lymphocytes, mRNAs induced in BL cells have been cloned and identified by subtractive hybridization. Nine genes encode RNAs which are 4- to > 100-fold more abundant after EBV infection. Two of these, the genes for CD21 and vimentin, were previously known to be induced by EBV infection. Five others, the genes for cathepsin H, annexin VI (p68), serglycin proteoglycan core protein, CD44, and the myristylated alanine-rich protein kinase C substrate (MARCKS), are genes which were not previously known to be induced by EBV infection. Two novel genes, EBV-induced genes 1 and 2 (EBI 1 and EBI 2, respectively) can be predicted from their cDNA sequences to encode G protein-coupled peptide receptors. EBI 1 is expressed exclusively in B- and T-lymphocyte cell lines and in lymphoid tissues and is highly homologous to the interleukin 8 receptors. EBI 2 is most closely related to the thrombin receptor. EBI 2 is expressed in B-lymphocyte cell lines and in lymphoid tissues but not in T-lymphocyte cell lines or peripheral blood T lymphocytes. EBI 2 is also expressed at lower levels in a promyelocytic and a histiocytic cell line and in pulmonary tissue. These predicted G protein-coupled peptide receptors are more likely to be mediators of EBV effects on B lymphocytes or of normal lymphocyte functions than are genes previously known to be up-regulated by EBV infection.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht J. C., Nicholas J., Biller D., Cameron K. R., Biesinger B., Newman C., Wittmann S., Craxton M. A., Coleman H., Fleckenstein B. Primary structure of the herpesvirus saimiri genome. J Virol. 1992 Aug;66(8):5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri C., Birkenbach M., Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991 Apr;181(2):595–608. doi: 10.1016/0042-6822(91)90893-g. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988 May;2(5):461–467. [PubMed] [Google Scholar]
- Barel M., Gauffre A., Lyamani F., Fiandino A., Hermann J., Frade R. Intracellular interaction of EBV/C3d receptor (CR2) with p68, a calcium-binding protein present in normal but not in transformed B lymphocytes. J Immunol. 1991 Aug 15;147(4):1286–1291. [PubMed] [Google Scholar]
- Biesinger B., Müller-Fleckenstein I., Simmer B., Lang G., Wittmann S., Platzer E., Desrosiers R. C., Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenbach M., Liebowitz D., Wang F., Sample J., Kieff E. Epstein-Barr virus latent infection membrane protein increases vimentin expression in human B-cell lines. J Virol. 1989 Sep;63(9):4079–4084. doi: 10.1128/jvi.63.9.4079-4084.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohley P., Seglen P. O. Proteases and proteolysis in the lysosome. Experientia. 1992 Feb 15;48(2):151–157. doi: 10.1007/BF01923508. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Anderson K. C., Freedman A. S., Fisher D. C., Slaughenhoupt B., Schlossman S. F., Nadler L. M. Studies of in vitro activation and differentiation of human B lymphocytes. I. Phenotypic and functional characterization of the B cell population responding to anti-Ig antibody. J Immunol. 1985 Mar;134(3):1516–1523. [PubMed] [Google Scholar]
- Brooks S. F., Herget T., Erusalimsky J. D., Rozengurt E. Protein kinase C activation potently down-regulates the expression of its major substrate, 80K, in Swiss 3T3 cells. EMBO J. 1991 Sep;10(9):2497–2505. doi: 10.1002/j.1460-2075.1991.tb07789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp R. L., Kraus T. A., Birkeland M. L., Puré E. High levels of CD44 expression distinguish virgin from antigen-primed B cells. J Exp Med. 1991 Mar 1;173(3):763–766. doi: 10.1084/jem.173.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Satchwell S. C., Preddie E., Weston K. M., Barrell B. G. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990 Apr 19;344(6268):774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- Clark D. M., Moss S. E., Wright N. A., Crumpton M. J. Expression of annexin VI (p68, 67 kDa-calelectrin) in normal human tissues: evidence for developmental regulation in B- and T-lymphocytes. Histochemistry. 1991;96(5):405–412. doi: 10.1007/BF00315998. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Kieff E. Epstein-Barr virus nuclear protein 2 mutations define essential domains for transformation and transactivation. J Virol. 1991 May;65(5):2545–2554. doi: 10.1128/jvi.65.5.2545-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier M., Calender A., Billaud M., Zimber U., Rousselet G., Pavlish O., Banchereau J., Tursz T., Bornkamm G., Lenoir G. M. Stable transfection of Epstein-Barr virus (EBV) nuclear antigen 2 in lymphoma cells containing the EBV P3HR1 genome induces expression of B-cell activation molecules CD21 and CD23. J Virol. 1990 Mar;64(3):1002–1013. doi: 10.1128/jvi.64.3.1002-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnow K. M., Pascoe L., White P. C. Genetic analysis of the human type-1 angiotensin II receptor. Mol Endocrinol. 1992 Jul;6(7):1113–1118. doi: 10.1210/mend.6.7.1508224. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Ehlin-Henriksson B., Manneborg-Sandlund A., Klein G. Expression of B-cell-specific markers in different Burkitt lymphoma subgroups. Int J Cancer. 1987 Feb 15;39(2):211–218. doi: 10.1002/ijc.2910390215. [DOI] [PubMed] [Google Scholar]
- Favrot M. C., Maritaz O., Suzuki T., Cooper M., Philip I., Philip T., Lenoir G. EBV-negative and -positive Burkitt cell lines variably express receptors for B-cell activation and differentiation. Int J Cancer. 1986 Dec 15;38(6):901–906. doi: 10.1002/ijc.2910380618. [DOI] [PubMed] [Google Scholar]
- Fyfe G., Cebra-Thomas J. A., Mustain E., Davie J. M., Alley C. D., Nahm M. H. Subpopulations of B lymphocytes in germinal centers. J Immunol. 1987 Oct 1;139(7):2187–2194. [PubMed] [Google Scholar]
- Fåhraeus R., Jansson A., Ricksten A., Sjöblom A., Rymo L. Epstein-Barr virus-encoded nuclear antigen 2 activates the viral latent membrane protein promoter by modulating the activity of a negative regulatory element. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7390–7394. doi: 10.1073/pnas.87.19.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. R., Jakway J. P., DeFranco A. L. Involvement of a guanine-nucleotide-binding component in membrane IgM-stimulated phosphoinositide breakdown. J Immunol. 1987 Dec 1;139(11):3604–3613. [PubMed] [Google Scholar]
- Gordon J., Walker L., Guy G., Brown G., Rowe M., Rickinson A. Control of human B-lymphocyte replication. II. Transforming Epstein-Barr virus exploits three distinct viral signals to undermine three separate control points in B-cell growth. Immunology. 1986 Aug;58(4):591–595. [PMC free article] [PubMed] [Google Scholar]
- Graff J. M., Young T. N., Johnson J. D., Blackshear P. J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21818–21823. [PubMed] [Google Scholar]
- Gregory C. D., Edwards C. F., Milner A., Wiels J., Lipinski M., Rowe M., Tursz T., Rickinson A. B. Isolation of a normal B cell subset with a Burkitt-like phenotype and transformation in vitro with Epstein-Barr virus. Int J Cancer. 1988 Aug 15;42(2):213–220. doi: 10.1002/ijc.2910420212. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Rowe M., Rickinson A. B. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990 Jul;71(Pt 7):1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Tursz T., Edwards C. F., Tetaud C., Talbot M., Caillou B., Rickinson A. B., Lipinski M. Identification of a subset of normal B cells with a Burkitt's lymphoma (BL)-like phenotype. J Immunol. 1987 Jul 1;139(1):313–318. [PubMed] [Google Scholar]
- Guy G. R., Gordon J. Epstein-Barr virus and a tumour-promoting phorbol ester use similar mechanisms in the stimulation of human B-cell proliferation. Int J Cancer. 1989 Apr 15;43(4):703–708. doi: 10.1002/ijc.2910430427. [DOI] [PubMed] [Google Scholar]
- Harlan D. M., Graff J. M., Stumpo D. J., Eddy R. L., Jr, Shows T. B., Boyle J. M., Blackshear P. J. The human myristoylated alanine-rich C kinase substrate (MARCKS) gene (MACS). Analysis of its gene product, promoter, and chromosomal localization. J Biol Chem. 1991 Aug 5;266(22):14399–14405. [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein coupling of antigen receptor-stimulated polyphosphoinositide hydrolysis in B cells. J Immunol. 1988 May 1;140(9):3135–3139. [PubMed] [Google Scholar]
- Hazarika P., Kaetzel M. A., Sheldon A., Karin N. J., Fleischer S., Nelson T. E., Dedman J. R. Annexin VI is associated with calcium-sequestering organelles. J Cell Biochem. 1991 May;46(1):78–85. doi: 10.1002/jcb.240460112. [DOI] [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Lee J., Kuang W. J., Rice G. C., Wood W. I. Structure and functional expression of a human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- Hornbeck P., Nakabayashi H., Fowlkes B. J., Paul W. E., Kligman D. A major myristylated substrate of protein kinase C and protein kinase C itself are differentially regulated during murine B- and T-lymphocyte development and activation. Mol Cell Biol. 1989 Sep;9(9):3727–3735. doi: 10.1128/mcb.9.9.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck P., Paul W. E. Anti-immunoglobulin and phorbol ester induce phosphorylation of proteins associated with the plasma membrane and cytoskeleton in murine B lymphocytes. J Biol Chem. 1986 Nov 5;261(31):14817–14824. [PubMed] [Google Scholar]
- Julius D., Livelli T. J., Jessell T. M., Axel R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989 Jun 2;244(4908):1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- Kalomiris E. L., Bourguignon L. Y. Mouse T lymphoma cells contain a transmembrane glycoprotein (GP85) that binds ankyrin. J Cell Biol. 1988 Feb;106(2):319–327. doi: 10.1083/jcb.106.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenton P., Johnson P. M., Webb P. D. The phosphorylation of p68, a calcium-binding protein associated with the human syncytiotrophoblast submembranous cytoskeleton, is modulated by growth factors, activators of protein kinase C and cyclic AMP. Biochim Biophys Acta. 1989 Dec 14;1014(3):271–281. doi: 10.1016/0167-4889(89)90223-1. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Knutson J. C. The level of c-fgr RNA is increased by EBNA-2, an Epstein-Barr virus gene required for B-cell immortalization. J Virol. 1990 Jun;64(6):2530–2536. doi: 10.1128/jvi.64.6.2530-2536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset S. O., Gallagher J. T. Proteoglycans in haemopoietic cells. Biochim Biophys Acta. 1990 Dec 11;1032(2-3):191–211. doi: 10.1016/0304-419x(90)90004-k. [DOI] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- König B., Arendt A., McDowell J. H., Kahlert M., Hargrave P. A., Hofmann K. P. Three cytoplasmic loops of rhodopsin interact with transducin. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6878–6882. doi: 10.1073/pnas.86.18.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz D., Kopan R., Fuchs E., Sample J., Kieff E. An Epstein-Barr virus transforming protein associates with vimentin in lymphocytes. Mol Cell Biol. 1987 Jul;7(7):2299–2308. doi: 10.1128/mcb.7.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. C., Südhof T. C., Anderson R. G. Annexin VI is required for budding of clathrin-coated pits. Cell. 1992 Jul 24;70(2):283–291. doi: 10.1016/0092-8674(92)90102-i. [DOI] [PubMed] [Google Scholar]
- Maciejewski J. P., Bruening E. E., Donahue R. E., Mocarski E. S., Young N. S., St Jeor S. C. Infection of hematopoietic progenitor cells by human cytomegalovirus. Blood. 1992 Jul 1;80(1):170–178. [PubMed] [Google Scholar]
- Matsumoto A. K., Kopicky-Burd J., Carter R. H., Tuveson D. A., Tedder T. F., Fearon D. T. Intersection of the complement and immune systems: a signal transduction complex of the B lymphocyte-containing complement receptor type 2 and CD19. J Exp Med. 1991 Jan 1;173(1):55–64. doi: 10.1084/jem.173.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey D. J., Nicotera P., Hartzell P., Bellomo G., Wyllie A. H., Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989 Feb 15;269(1):365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- McIlroy B. K., Walters J. D., Blackshear P. J., Johnson J. D. Phosphorylation-dependent binding of a synthetic MARCKS peptide to calmodulin. J Biol Chem. 1991 Mar 15;266(8):4959–4964. [PubMed] [Google Scholar]
- Moser B., Schumacher C., von Tscharner V., Clark-Lewis I., Baggiolini M. Neutrophil-activating peptide 2 and gro/melanoma growth-stimulatory activity interact with neutrophil-activating peptide 1/interleukin 8 receptors on human neutrophils. J Biol Chem. 1991 Jun 5;266(16):10666–10671. [PubMed] [Google Scholar]
- Murphy P. M., Tiffany H. L. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- Nicholas J., Cameron K. R., Honess R. W. Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature. 1992 Jan 23;355(6358):362–365. doi: 10.1038/355362a0. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Klein G., Henle W., Henle G. The establishment of lymphoblastoid lines from adult and fetal human lymphoid tissue and its dependence on EBV. Int J Cancer. 1971 Nov 15;8(3):443–450. doi: 10.1002/ijc.2910080312. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Klein G. Phenotypic and cytogenetic characteristics of human B-lymphoid cell lines and their relevance for the etiology of Burkitt's lymphoma. Adv Cancer Res. 1982;37:319–380. doi: 10.1016/s0065-230x(08)60886-6. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Rimland J., Xin W., Sweetnam P., Saijoh K., Nestler E. J., Duman R. S. Sequence and expression of a neuropeptide Y receptor cDNA. Mol Pharmacol. 1991 Dec;40(6):869–875. [PubMed] [Google Scholar]
- Rosen A., Keenan K. F., Thelen M., Nairn A. C., Aderem A. Activation of protein kinase C results in the displacement of its myristoylated, alanine-rich substrate from punctate structures in macrophage filopodia. J Exp Med. 1990 Oct 1;172(4):1211–1215. doi: 10.1084/jem.172.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rooney C. M., Edwards C. F., Lenoir G. M., Rickinson A. B. Epstein-Barr virus status and tumour cell phenotype in sporadic Burkitt's lymphoma. Int J Cancer. 1986 Mar 15;37(3):367–373. doi: 10.1002/ijc.2910370307. [DOI] [PubMed] [Google Scholar]
- Rowe M., Rooney C. M., Rickinson A. B., Lenoir G. M., Rupani H., Moss D. J., Stein H., Epstein M. A. Distinctions between endemic and sporadic forms of Epstein-Barr virus-positive Burkitt's lymphoma. Int J Cancer. 1985 Apr 15;35(4):435–441. doi: 10.1002/ijc.2910350404. [DOI] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991 Feb 15;251(4995):804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- Spira G., Aman P., Koide N., Lundin G., Klein G., Hall K. Cell-surface immunoglobulin and insulin receptor expression in an EBV-negative lymphoma cell line and its EBV-converted sublines. J Immunol. 1981 Jan;126(1):122–126. [PubMed] [Google Scholar]
- Sreedharan S. P., Robichon A., Peterson K. E., Goetzl E. J. Cloning and expression of the human vasoactive intestinal peptide receptor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4986–4990. doi: 10.1073/pnas.88.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Expression of cell surface markers after human B lymphocyte activation. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3848–3852. doi: 10.1073/pnas.78.6.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Candelore M. R., Rands E., Hill W. S., Dixon R. A. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988 Jul 25;263(21):10267–10271. [PubMed] [Google Scholar]
- Strosberg A. D. Structure/function relationship of proteins belonging to the family of receptors coupled to GTP-binding proteins. Eur J Biochem. 1991 Feb 26;196(1):1–10. doi: 10.1111/j.1432-1033.1991.tb15778.x. [DOI] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Sanders S. K., Butler J. L., Gartland G. L., Komiyama K., Cooper M. D. Identification of an early activation antigen (Bac-1) on human B cells. J Immunol. 1986 Aug 15;137(4):1208–1213. [PubMed] [Google Scholar]
- Tanner J., Weis J., Fearon D., Whang Y., Kieff E. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell. 1987 Jul 17;50(2):203–213. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- Thelen M., Rosen A., Nairn A. C., Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991 May 23;351(6324):320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Nadler L. M., Bhan A. K., Schooley R. T. BLAST-2 [EBVCS], an early cell surface marker of human B cell activation, is superinduced by Epstein Barr virus. J Immunol. 1985 May;134(5):3007–3012. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Swendeman S. L., Edson C. M. Biochemical analysis suggests distinct functional roles for the BLAST-1 and BLAST-2 antigens. J Immunol. 1986 Mar 1;136(5):1745–1751. [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. The truncated form of the Epstein-Barr virus latent-infection membrane protein expressed in virus replication does not transform rodent fibroblasts. J Virol. 1988 Jul;62(7):2337–2346. doi: 10.1128/jvi.62.7.2337-2346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Tsang S. F., Kurilla M. G., Cohen J. I., Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J Virol. 1990 Jul;64(7):3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J. J., Tedder T. F., Fearon D. T. Identification of a 145,000 Mr membrane protein as the C3d receptor (CR2) of human B lymphocytes. Proc Natl Acad Sci U S A. 1984 Feb;81(3):881–885. doi: 10.1073/pnas.81.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]