Abstract
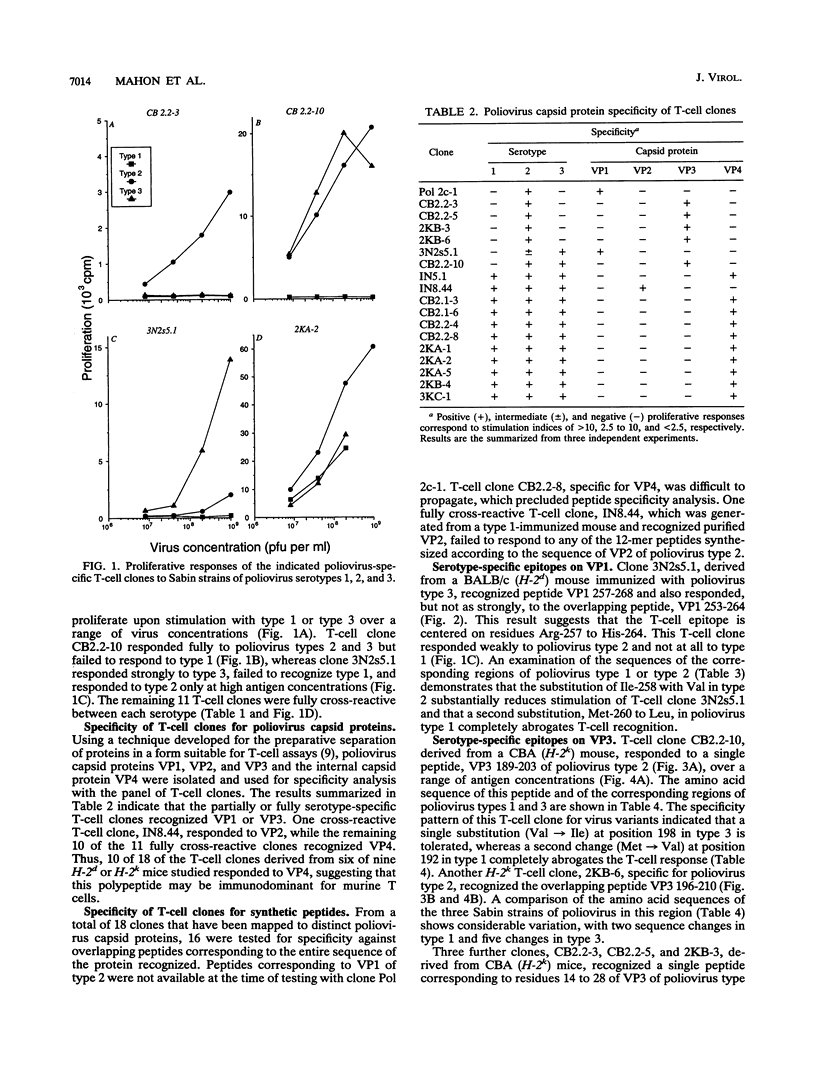
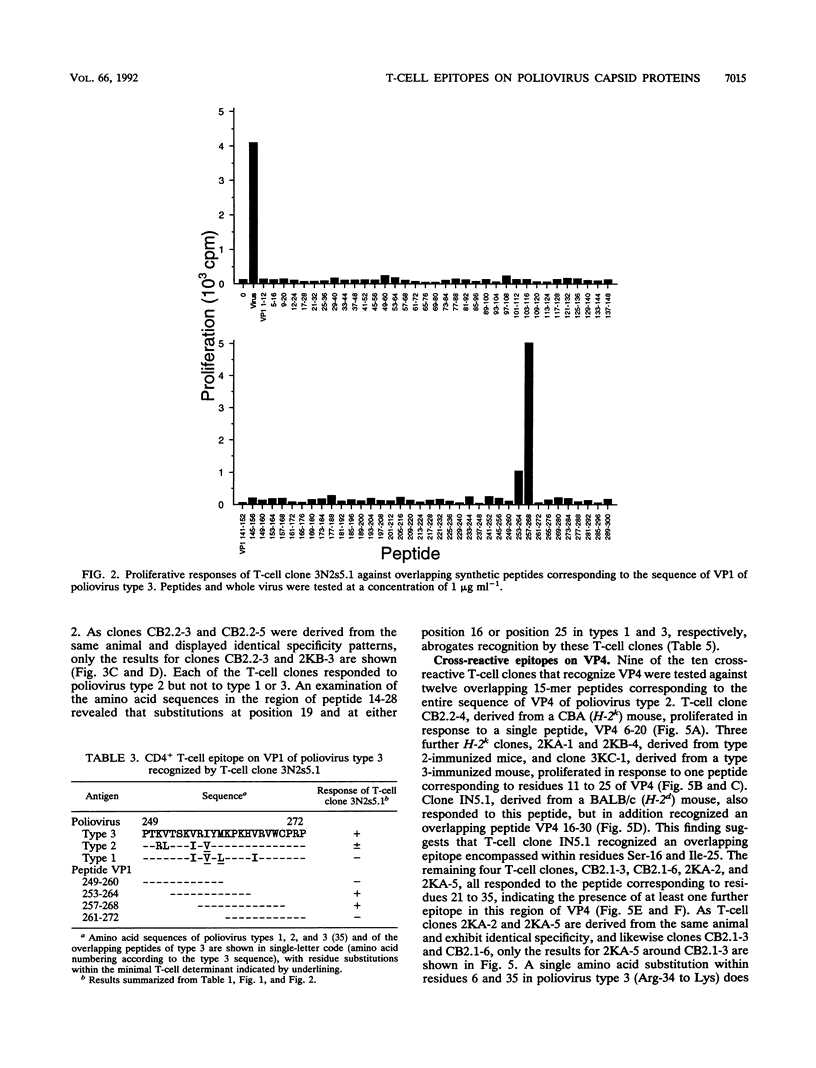
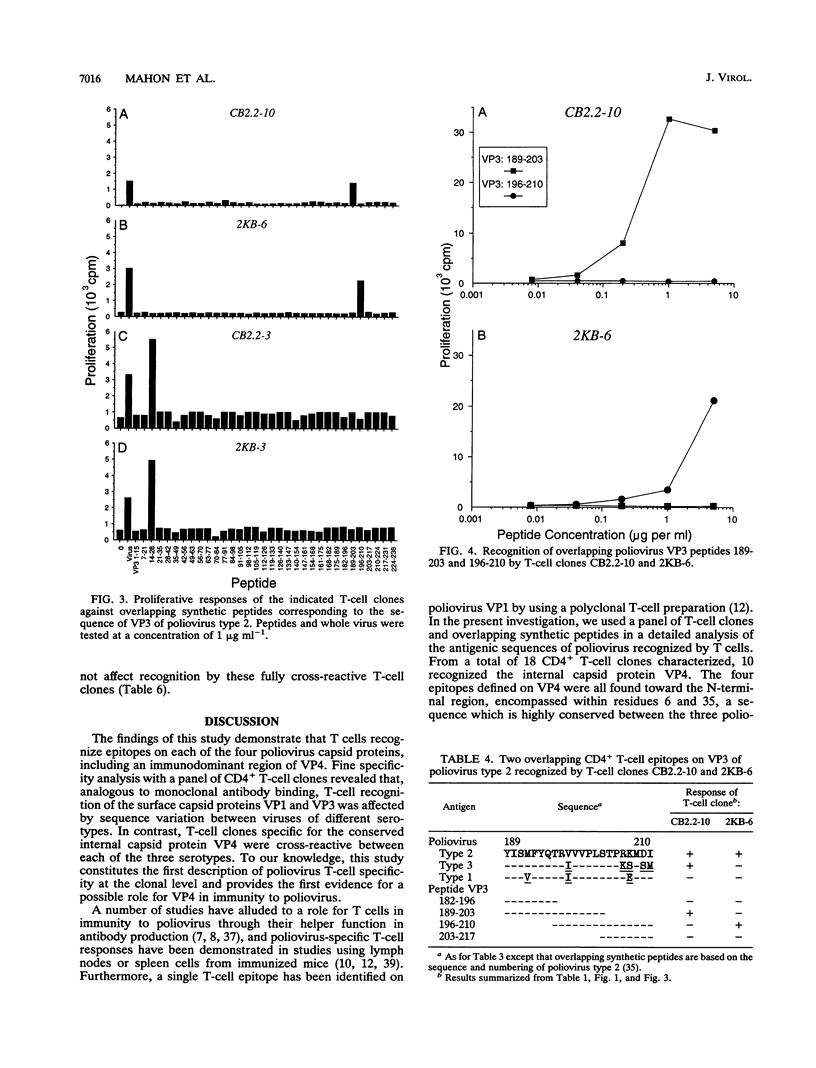
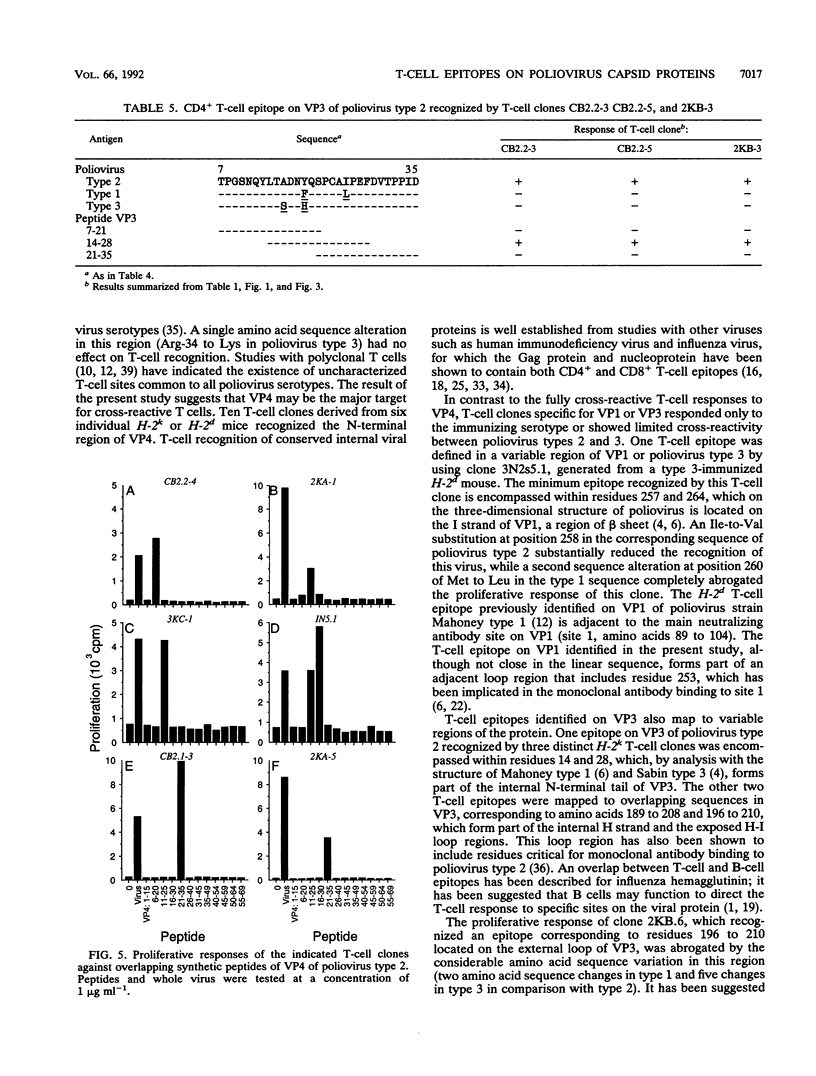
A panel of poliovirus-specific murine CD4+ T-cell clones has been established from both BALB/c (H-2d) and CBA (H-2k) mice immunized with Sabin vaccine strains of poliovirus serotype 1, 2, or 3. T-cell clones were found to be either serotype specific or cross-reactive between two or all three serotypes. Specificity analysis against purified poliovirus proteins demonstrated that T-cell clones recognized determinants on the surface capsid proteins VP1, VP2, and VP3 and the internal capsid protein VP4. Panels of overlapping synthetic peptides were used to identify eight distinct T-cell epitopes. One type 3-specific T-cell clone recognized an epitope within amino acids 257 and 264 of VP1. Three T-cell epitopes corresponding to residues 14 to 28, 189 to 203, and 196 to 210 were identified on VP3 of poliovirus type 2. The remaining four T-cell epitopes were mapped to an immunodominant region of VP4, encompassed within residues 6 and 35 and recognized by both H-2d and H-2k mice. The epitopes on VP4 were conserved between serotypes, and this may account for the predominantly cross-reactive poliovirus-specific T-cell response observed with polyclonal T-cell populations. In contrast, T-cell clones that recognize epitopes on VP1 or VP3 were largely serotype specific; single or multiple amino acid substitutions were found to be critical for T-cell recognition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett B. C., Graham C. M., Burt D. S., Skehel J. J., Thomas D. B. The immune response of BALB/c mice to influenza hemagglutinin: commonality of the B cell and T cell repertoires and their relevance to antigenic drift. Eur J Immunol. 1989 Mar;19(3):515–521. doi: 10.1002/eji.1830190316. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Nygard N. R., Graham M. B., Bono C., Braciale V. L., Gorka J., Schwartz B. D., Braciale T. J. Recognition of the influenza hemagglutinin by class II MHC-restricted T lymphocytes and antibodies. I. Site definition and implications for antigen presentation and T lymphocyte recognition. J Immunol. 1991 Oct 15;147(8):2677–2684. [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Filman D. J., Syed R., Chow M., Macadam A. J., Minor P. D., Hogle J. M. Structural factors that control conformational transitions and serotype specificity in type 3 poliovirus. EMBO J. 1989 May;8(5):1567–1579. doi: 10.1002/j.1460-2075.1989.tb03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W. P., McCollough R. H., Lamb G. A., Chin T. D. Quantitative relationship of preexisting homotypic antibodies to excretion of poliovirus types 1, 2, and 3 following the feeding of trivalent attenuated poliovirus vaccine. Am J Epidemiol. 1969 Aug;90(2):146–156. doi: 10.1093/oxfordjournals.aje.a121058. [DOI] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Icenogle J. P., Minor P. D., Ferguson M., Hogle J. M. Modulation of humoral response to a 12-amino-acid site on the poliovirus virion. J Virol. 1986 Oct;60(1):297–301. doi: 10.1128/jvi.60.1.297-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubelt B., Ropka S. L., Goldfarb S. J., Janavs J. L. Anti-thymocyte serum delays clearance of poliovirus from the mouse central nervous system. J Neuroimmunol. 1989 May;22(3):223–232. doi: 10.1016/0165-5728(89)90020-9. [DOI] [PubMed] [Google Scholar]
- Katrak K., Mahon B. P., Minor P. D., Mills K. H. Cellular and humoral immune responses to poliovirus in mice: a role for helper T cells in heterotypic immunity to poliovirus. J Gen Virol. 1991 May;72(Pt 5):1093–1098. doi: 10.1099/0022-1317-72-5-1093. [DOI] [PubMed] [Google Scholar]
- Koike S., Taya C., Kurata T., Abe S., Ise I., Yonekawa H., Nomoto A. Transgenic mice susceptible to poliovirus. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):951–955. doi: 10.1073/pnas.88.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc C., Deriaud E., Mimic V., van der Werf S. Identification of a T-cell epitope adjacent to neutralization antigenic site 1 of poliovirus type 1. J Virol. 1991 Feb;65(2):711–718. doi: 10.1128/jvi.65.2.711-718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley M. D., Thiemann R., Rodriguez M. Cytotoxic T cells isolated from the central nervous systems of mice infected with Theiler's virus. J Virol. 1991 Dec;65(12):6612–6620. doi: 10.1128/jvi.65.12.6612-6620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeji N. J., Bray A. M., Geysen H. M. Multi-pin peptide synthesis strategy for T cell determinant analysis. J Immunol Methods. 1990 Nov 6;134(1):23–33. doi: 10.1016/0022-1759(90)90108-8. [DOI] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Thornton G. B., Hughes J. L. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987 Oct 8;329(6139):547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- Mills K. H., Barnard A. L., Williams M., Page M., Ling C., Stott E. J., Silvera P., Taffs F., Kingsman A. S., Adams S. E. Vaccine-induced CD4+ T cells against the simian immunodeficiency virus gag protein. Epitope specificity and relevance to protective immunity. J Immunol. 1991 Nov 15;147(10):3560–3567. [PubMed] [Google Scholar]
- Mills K. H., Kitchin P. A., Mahon B. P., Barnard A. L., Adams S. E., Kingsman S. M., Kingsman A. J. HIV p24-specific helper T cell clones from immunized primates recognize highly conserved regions of HIV-1. J Immunol. 1990 Mar 1;144(5):1677–1683. [PubMed] [Google Scholar]
- Mills K. H., Skehel J. J., Thomas D. B. Extensive diversity in the recognition of influenza virus hemagglutinin by murine T helper clones. J Exp Med. 1986 Jun 1;163(6):1477–1490. doi: 10.1084/jem.163.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Ferguson M., Evans D. M., Almond J. W., Icenogle J. P. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J Gen Virol. 1986 Jul;67(Pt 7):1283–1291. doi: 10.1099/0022-1317-67-7-1283. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Muller D., Koller B. H., Whitton J. L., LaPan K. E., Brigman K. K., Frelinger J. A. LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science. 1992 Mar 20;255(5051):1576–1578. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- Nixon D. F., Townsend A. R., Elvin J. G., Rizza C. R., Gallwey J., McMichael A. J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988 Dec 1;336(6198):484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- Palladino G., Scherle P. A., Gerhard W. Activity of CD4+ T-cell clones of type 1 and type 2 in generation of influenza virus-specific cytotoxic responses in vitro. J Virol. 1991 Nov;65(11):6071–6076. doi: 10.1128/jvi.65.11.6071-6076.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena Rossi C., McAllister A., Fiette L., Brahic M. Theiler's virus infection induces a specific cytotoxic T lymphocyte response. Cell Immunol. 1991 Dec;138(2):341–348. doi: 10.1016/0008-8749(91)90158-8. [DOI] [PubMed] [Google Scholar]
- Phillips R. E., Rowland-Jones S., Nixon D. F., Gotch F. M., Edwards J. P., Ogunlesi A. O., Elvin J. G., Rothbard J. A., Bangham C. R., Rizza C. R. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991 Dec 12;354(6353):453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- Pircher H., Moskophidis D., Rohrer U., Bürki K., Hengartner H., Zinkernagel R. M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990 Aug 16;346(6285):629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Ren R. B., Costantini F., Gorgacz E. J., Lee J. J., Racaniello V. R. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell. 1990 Oct 19;63(2):353–362. doi: 10.1016/0092-8674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986 Oct 1;164(4):1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tite J. P., Russell S. M., Dougan G., O'Callaghan D., Jones I., Brownlee G., Liew F. Y. Antiviral immunity induced by recombinant nucleoprotein of influenza A virus. I. Characteristics and cross-reactivity of T cell responses. J Immunol. 1988 Dec 1;141(11):3980–3987. [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Kohara M., Kataoka Y., Suganuma T., Omata T., Imura N., Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984 Apr 25;174(4):561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- Uhlig J., Wiegers K., Dernick R. A new antigenic site of poliovirus recognized by an intertypic cross-neutralizing monoclonal antibody. Virology. 1990 Oct;178(2):606–610. doi: 10.1016/0042-6822(90)90363-v. [DOI] [PubMed] [Google Scholar]
- Uytdehaag F. G., Loggen H. G., Logtenberg T., Lichtveld R. A., van Steenis B., van Asten J. A., Osterhaus A. D. Human peripheral blood lymphocytes from recently vaccinated individuals produce both type-specific and intertypic cross-reacting neutralizing antibody on in vitro stimulation with one type of poliovirus. J Immunol. 1985 Nov;135(5):3094–3101. [PubMed] [Google Scholar]
- Wang K. G., Sun L. Z., Jubelt B., Waltenbaugh C. Cell-mediated immune responses to poliovirus. I. Conditions for induction, characterization of effector cells, and cross-reactivity between serotypes for delayed hypersensitivity and T cell proliferative responses. Cell Immunol. 1989 Apr 1;119(2):252–262. doi: 10.1016/0008-8749(89)90242-6. [DOI] [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus tb-3 infection. II. Characterization of effector cells and demonstration cytotoxicity against viral-infected myofibers1. J Immunol. 1977 Apr;118(4):1165–1169. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]


