Abstract
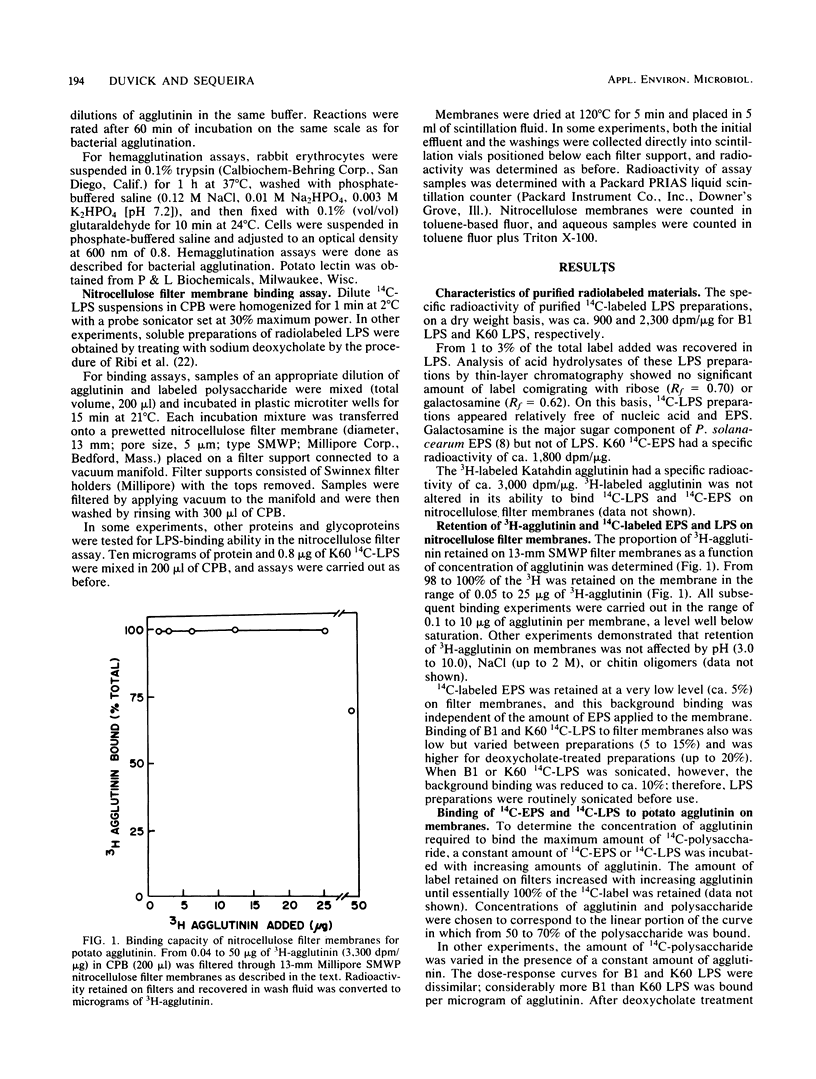
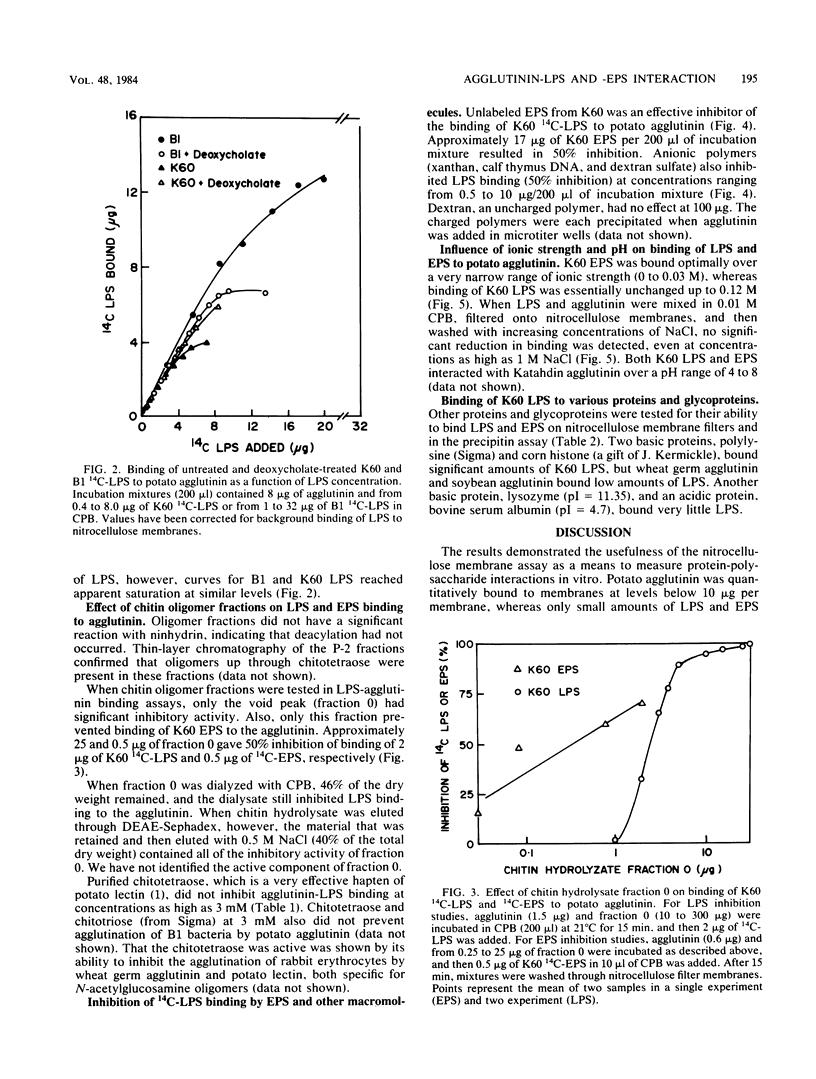
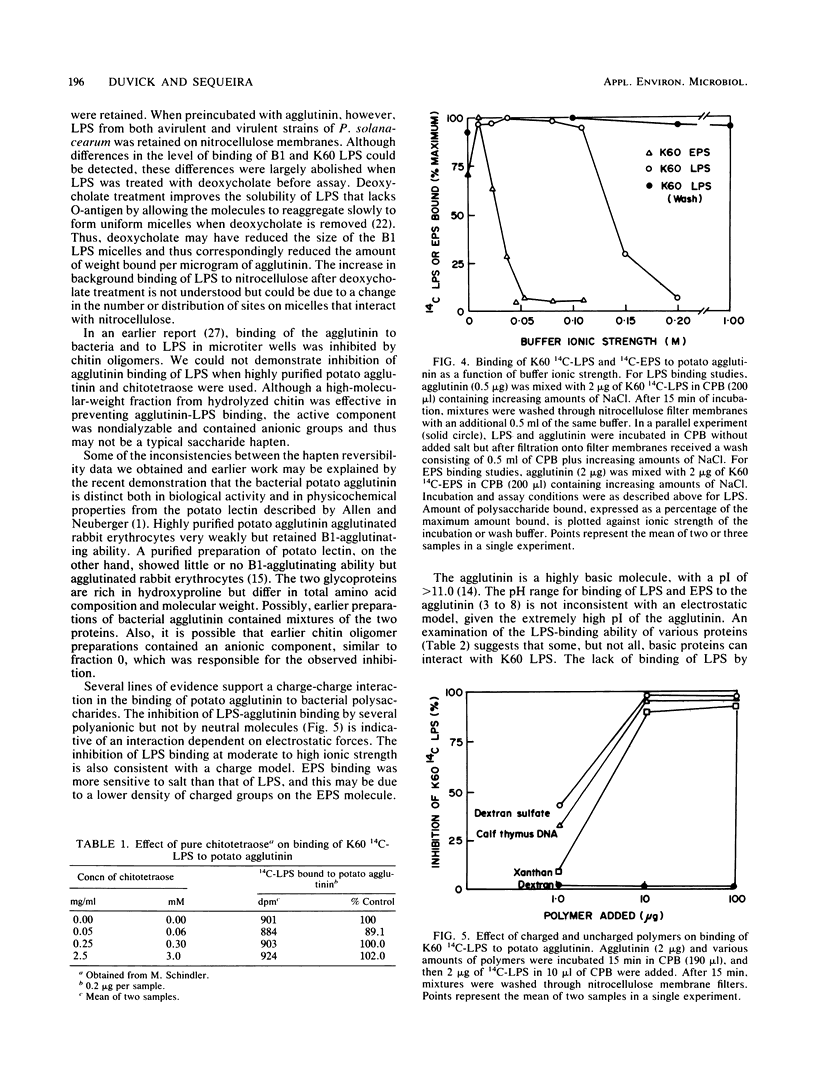
In vitro binding assays were used to study the possible role of a cell wall agglutinin in the attachment to plant cell walls of avirulent strains of the wilt pathogen, Pseudomonas solanacearum. In a nitrocellulose filter assay, radioactively labeled lipopolysaccharide (LPS) from the virulent strain, K60, and the avirulent strain, B1, and extracellular polysaccharide (EPS) from K60 were bound quantitatively by the agglutinin extracted from Katahdin potato tubers. The LPS from B1 had significantly greater agglutinin-binding affinity than that from K60 but not after treatment with deoxycholate, which improved solubility. Highly purified chitotetraose did not inhibit binding of K60 LPS to agglutinin, but binding was inhibited by EPS as well as by diverse anionic polymers (DNA, dextran sulfate, xanthan). Binding of agglutinin to EPS and LPS was inhibited at ionic strengths greater than 0.03 and 0.15 M, respectively. It was concluded that electrostatic charge-charge interactions could account for binding of LPS and EPS to potato agglutinin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Neuberger A. The purification and properties of the lectin from potato tubers, a hydroxyproline-containing glycoprotein. Biochem J. 1973 Oct;135(2):307–314. doi: 10.1042/bj1350307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradshaw-Rouse J. J., Whatley M. H., Coplin D. L., Woods A., Sequeira L., Kelman A. Agglutination of Erwinia stewartii Strains with a Corn Agglutinin: Correlation with Extracellular Polysaccharide Production and Pathogenicity. Appl Environ Microbiol. 1981 Aug;42(2):344–350. doi: 10.1128/aem.42.2.344-350.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDMAN W. F. Comparison of slime from tomato and banana strains of Pseudomonas solanacearum. Nature. 1959 Dec 19;184(Suppl 25):1969–1970. doi: 10.1038/1841969a0. [DOI] [PubMed] [Google Scholar]
- Dröge W., Lehmann V., Lüderitz O., Westphal O. Structural investigations on the 2-keto-3-deoxyoctonate region of lipopolysaccharides. Eur J Biochem. 1970 May 1;14(1):175–184. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Duvick J. P., Sequeira L. Interaction of Pseudomonas solanacearum with Suspension-Cultured Tobacco Cells and Tobacco Leaf Cell Walls In Vitro. Appl Environ Microbiol. 1984 Jul;48(1):199–205. doi: 10.1128/aem.48.1.199-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Sequeira L. A New Bacterial Agglutinin from Soybean: I. ISOLATION, PARTIAL PURIFICATION, AND CHARACTERIZATION. Plant Physiol. 1980 Nov;66(5):847–852. doi: 10.1104/pp.66.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Sequeira L. A New Bacterial Agglutinin from Soybean: II. EVIDENCE AGAINST A ROLE IN DETERMINING PATHOGEN SPECIFICITY. Plant Physiol. 1980 Nov;66(5):853–858. doi: 10.1104/pp.66.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J. E., Cantrell M. A., Sequeira L. Hydroxyproline-rich bacterial agglutinin from potato : extraction, purification, and characterization. Plant Physiol. 1982 Nov;70(5):1353–1358. doi: 10.1104/pp.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Debray H., Cacan M., Cacan R., Sharons N. Labeling of soybean agglutinin by oxidation with sodium periodate followed by reduction with sodium [3-H]borohydride. J Biol Chem. 1975 Mar 10;250(5):1955–1957. [PubMed] [Google Scholar]
- MARINKOVICH V. A. PURIFICATION AND CHARACTERIZATION OF THE HEMAGGLUTININ PRESENT IN POTATOES. J Immunol. 1964 Nov;93:732–741. [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- Mellon J. E., Helgeson J. P. Interaction of a hydroxyproline-rich glycoprotein from tobacco callus with potential pathogens. Plant Physiol. 1982 Aug;70(2):401–405. doi: 10.1104/pp.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Sene C., Maget-Dana R., Delmotte F. Sugar-lectin interactions: how does wheat-germ agglutinin bind sialoglycoconjugates? Eur J Biochem. 1980 Feb;104(1):147–153. doi: 10.1111/j.1432-1033.1980.tb04410.x. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brown R., Haskins W. T., Malmgren B., Milner K. C., Rudbach J. A. Reaction of endotoxin and surfactants. I. Physical and biological properties of endotoxin treated with sodium deoxycholate. J Bacteriol. 1966 Nov;92(5):1493–1509. doi: 10.1128/jb.92.5.1493-1509.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLONEKER J. H., ORENTAS D. G. Pyruvic acid, a unique component of an exocellular bacterial polysaccharide. Nature. 1962 May 5;194:478–479. doi: 10.1038/194478a0. [DOI] [PubMed] [Google Scholar]
- Weckesser J., Mayer H., Drews G., Fromme I. Lipophilic O-antigens containing D-glycero-D-mannoheptose as the sole neutral sugar in Rhodopseudomonas gelatinosa. J Bacteriol. 1975 Aug;123(2):449–455. doi: 10.1128/jb.123.2.449-455.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley M. H., Bodwin J. S., Lippincott B. B., Lippincott J. A. Role of Agrobacterium cell envelope lipopolysaccharide in infection site attachment. Infect Immun. 1976 Apr;13(4):1080–1083. doi: 10.1128/iai.13.4.1080-1083.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley M. H., Hunter N., Cantrell M. A., Hendrick C., Keegstra K., Sequeira L. Lipopolysaccharide Composition of the Wilt Pathogen, Pseudomonas solanacearum: CORRELATION WITH THE HYPERSENSITIVE RESPONSE IN TOBACCO. Plant Physiol. 1980 Mar;65(3):557–559. doi: 10.1104/pp.65.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]


