Abstract
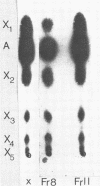
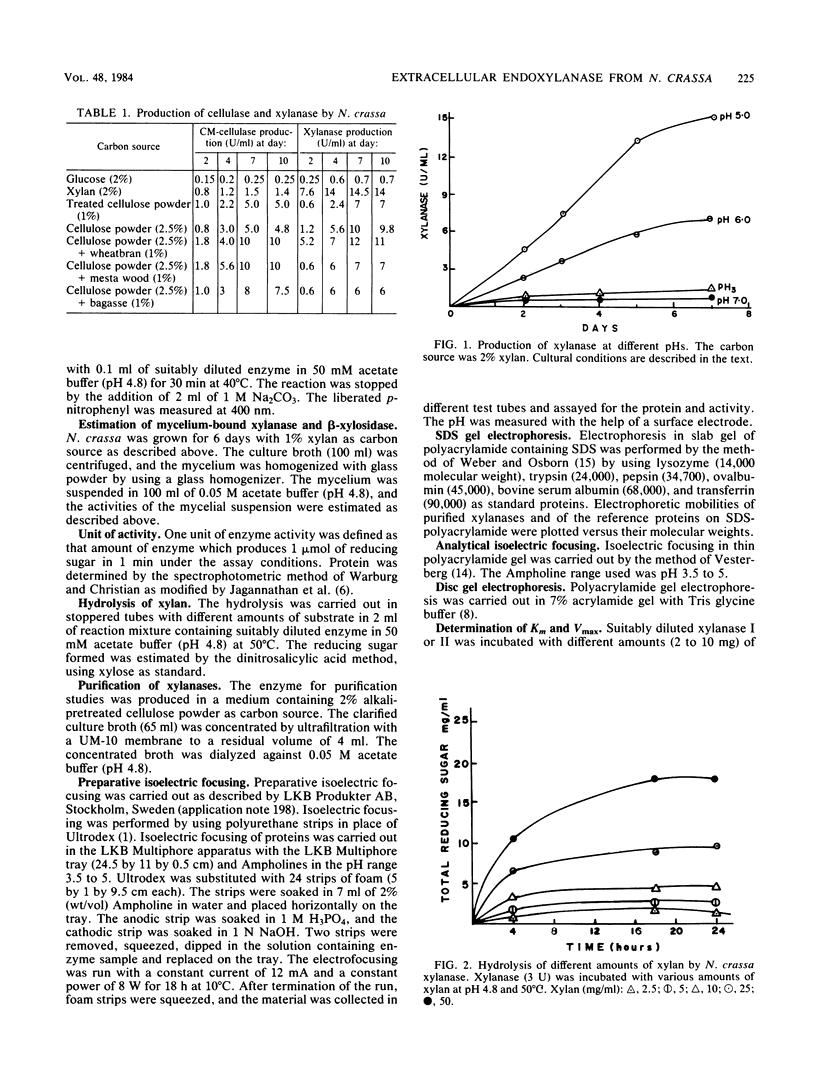
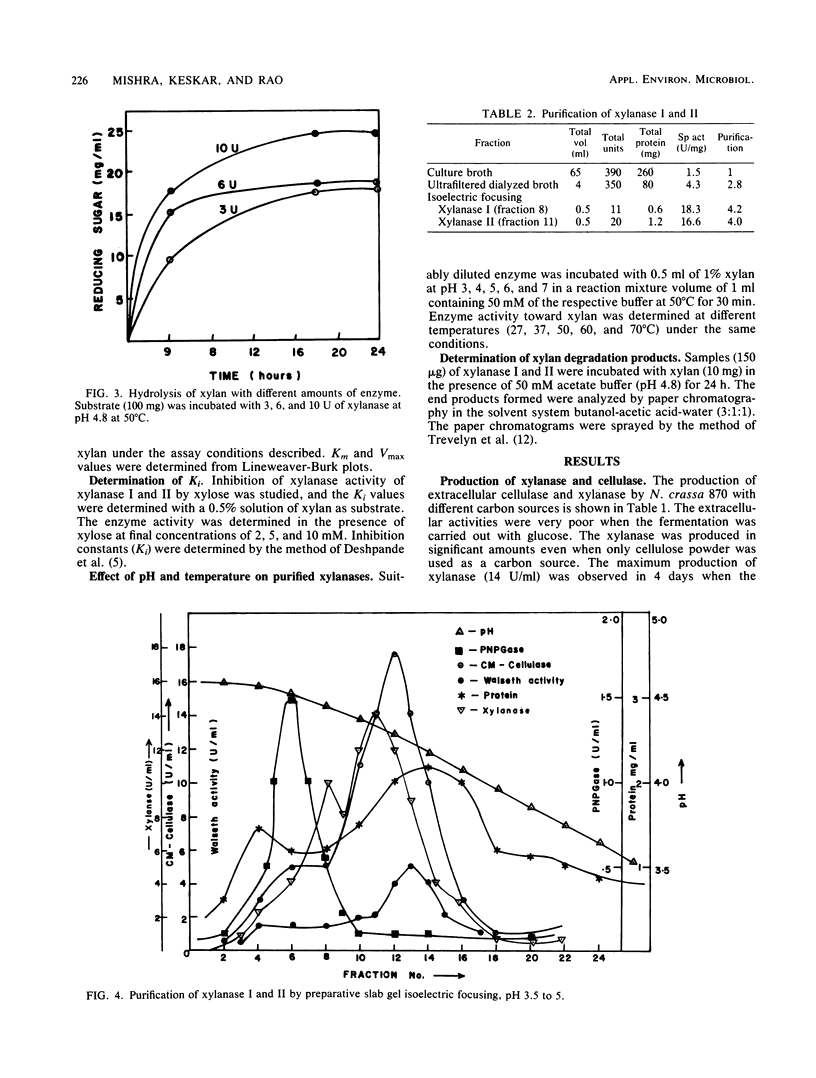
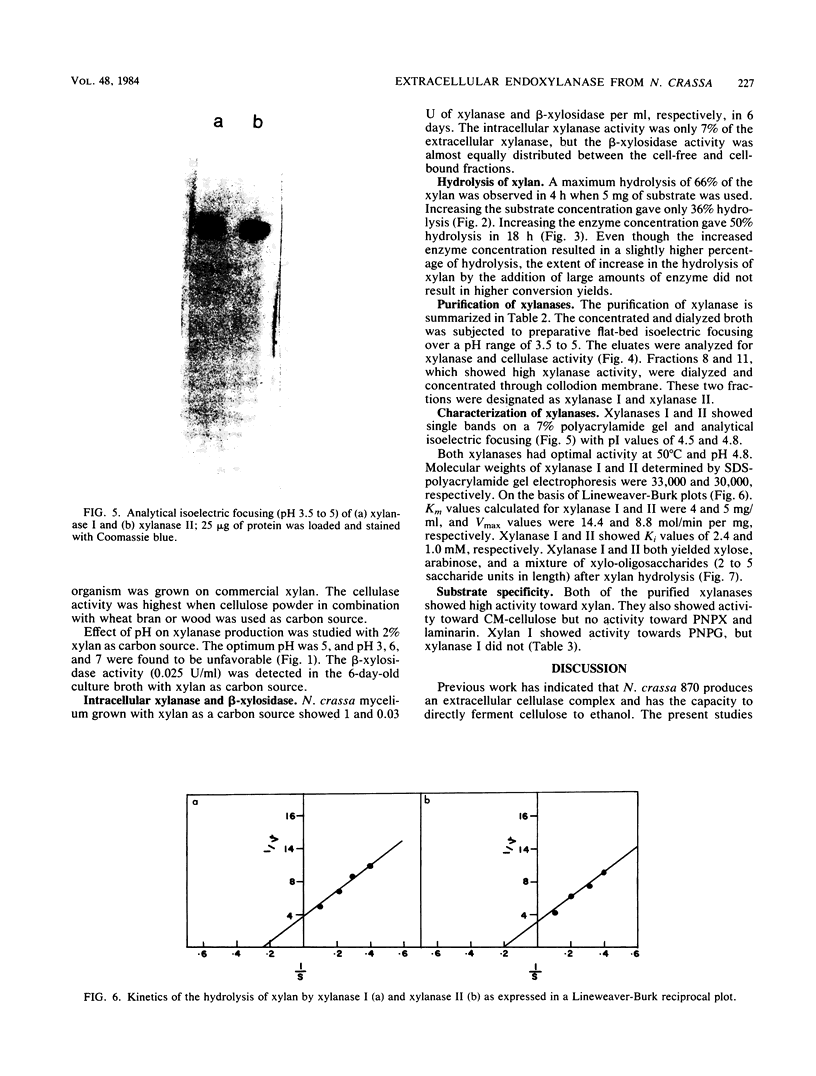
Neurospora crassa 870 produced 14 and 0.025 U of extracellular xylanase (1,4-β-d-xylan xylanohydrolase; EC 3.2.1.8) and β-xylosidase (1,4-β-xylan xylohydrolase; EC 3.2.1.37) per ml, respectively, in 4 days when commercial xylan was used as a carbon source. The effects of pH and carbon sources on xylanase production by N. crassa are discussed. Two xylanases (I and II) were purified and had pI values of 4.8 and 4.5 and molecular weights of 33,000 and 30,000. The maximum degree of hydrolysis of xylan by the extracellular culture broth was 66% in 4 h. The end products of xylan hydrolysis by xylanase I and II showed the presence of xylose, xylobiose, xylotriose, xylotetraose, xylopentose, and arabinose, indicating that they are endoxylanases capable of hydrolyzing 1,3-α-l-arabinofuranosyl branch points. Both xylanases showed activity toward carboxymethyl cellulose but no activity toward para-nitrophenyl-β-d-xyloside or laminarin. Xylanase I showed appreciable activity toward para-nitrophenyl-β-d-glucoside, whereas xylanase II was inactive.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodhe A. M., Deshpande V. V., Lakshmikantham B. C., Vartak H. G. Simplified techniques for elution of proteins from polyacrylamide gel, staining, destaining, and isoelectric focusing. Anal Biochem. 1982 Jun;123(1):133–142. doi: 10.1016/0003-2697(82)90633-9. [DOI] [PubMed] [Google Scholar]
- Bucht B., Eriksson K. E. Extracellular enzyme system utilized by the rot fungus Stereum sanguinolentum for the breakdown of cellulose. I. Studies on the enzyme production. Arch Biochem Biophys. 1968 Mar 20;124(1):135–141. doi: 10.1016/0003-9861(68)90312-3. [DOI] [PubMed] [Google Scholar]
- Dekker R. F., Richards G. N. Hemicellulases: their occurrence, purification, properties, and mode of action. Adv Carbohydr Chem Biochem. 1976;32:277–352. doi: 10.1016/s0065-2318(08)60339-x. [DOI] [PubMed] [Google Scholar]
- Deshpande V., Eriksson K. E., Pettersson B. Production , purification and partial characterization of 1,4-beta-glucosidase enzymes from Sporotrichum pulverulentum. Eur J Biochem. 1978 Sep 15;90(1):191–198. doi: 10.1111/j.1432-1033.1978.tb12590.x. [DOI] [PubMed] [Google Scholar]
- TIMELL T. E. WOOD HEMICELLULOSES. I. Adv Carbohydr Chem. 1964;19:247–302. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]