Abstract
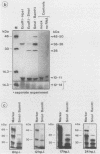
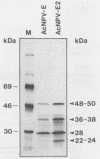
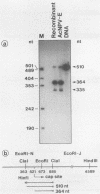
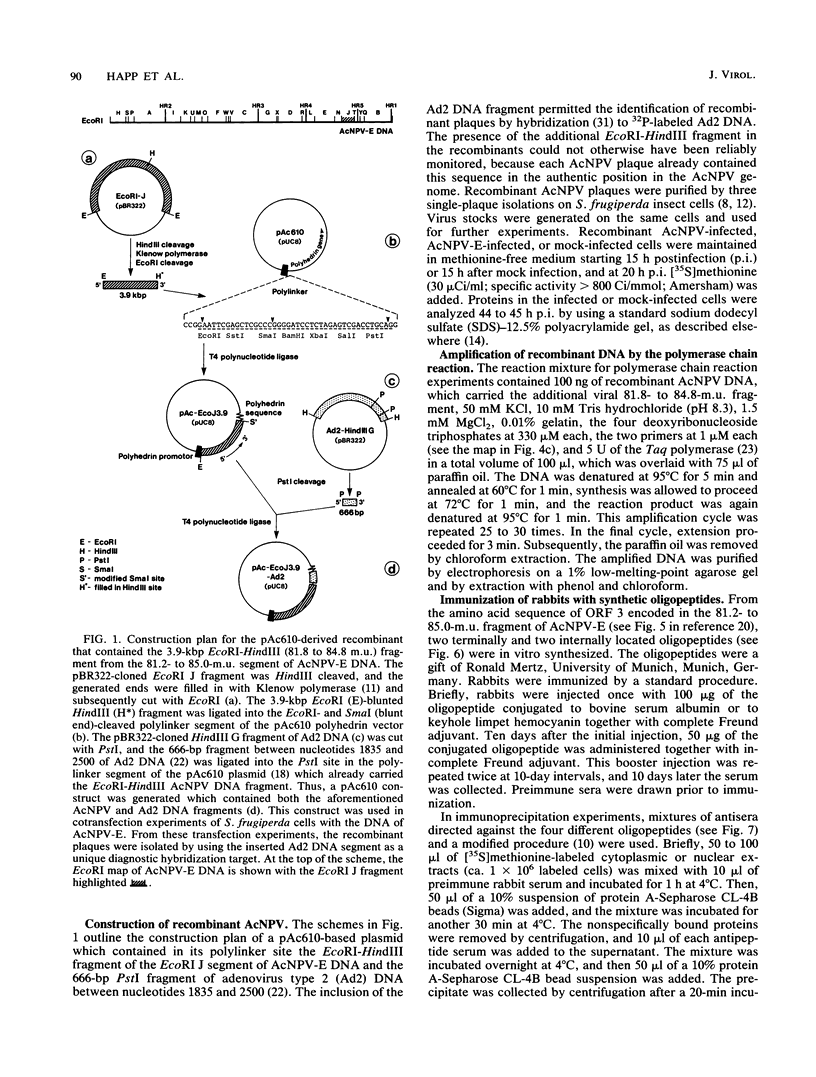
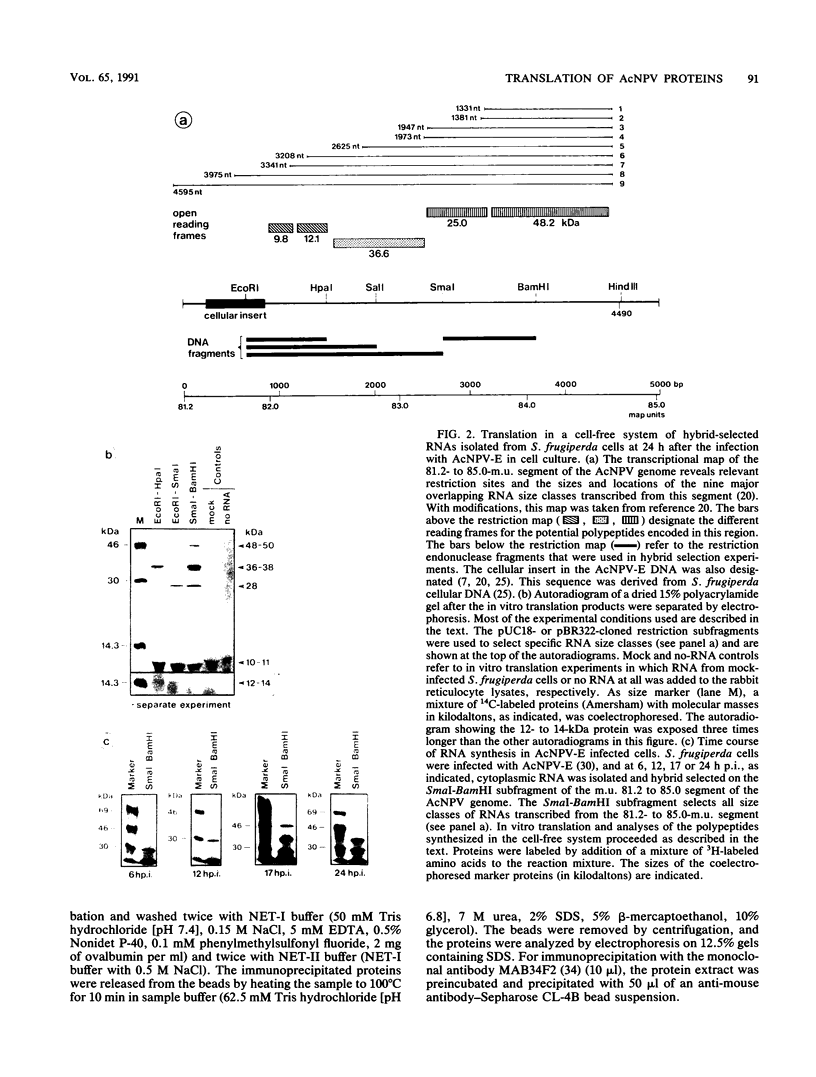
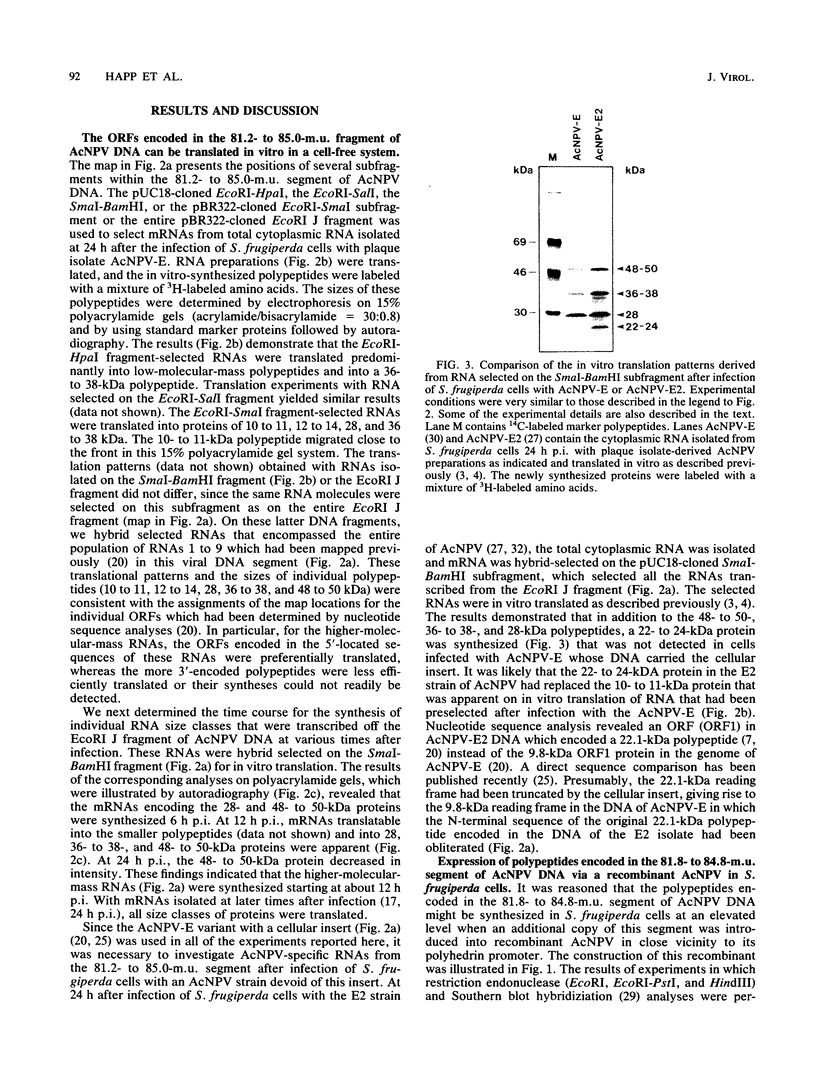
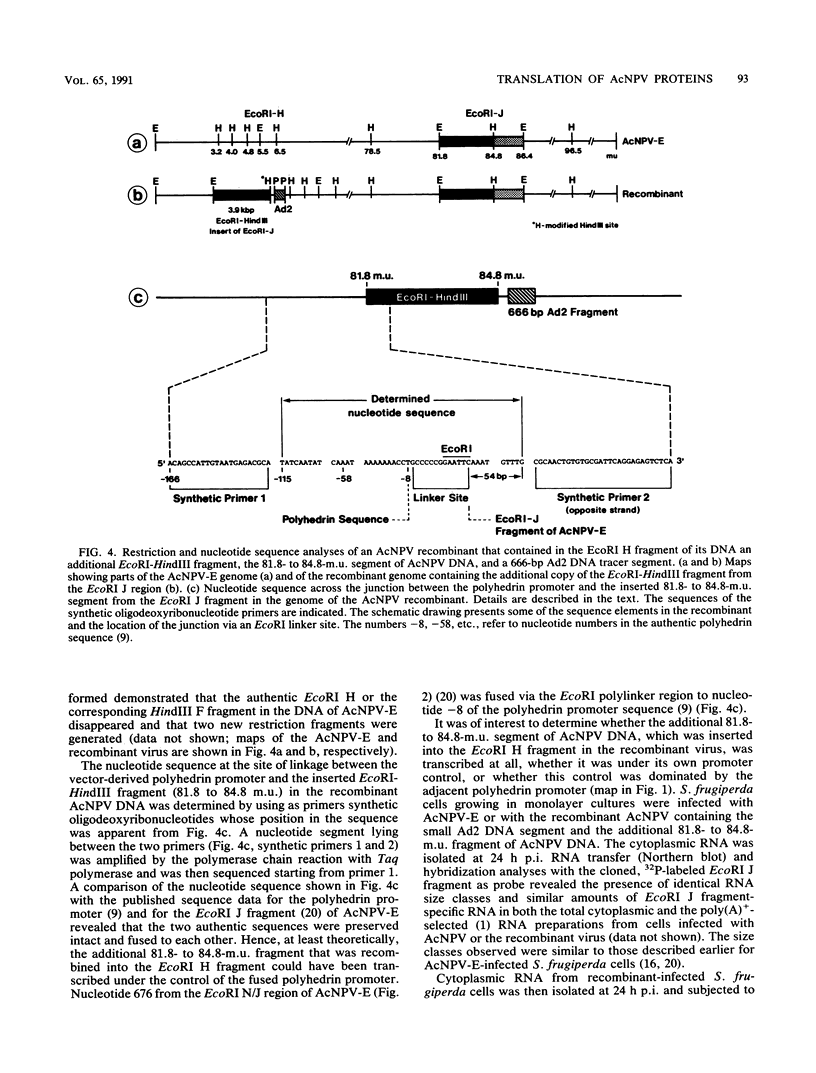
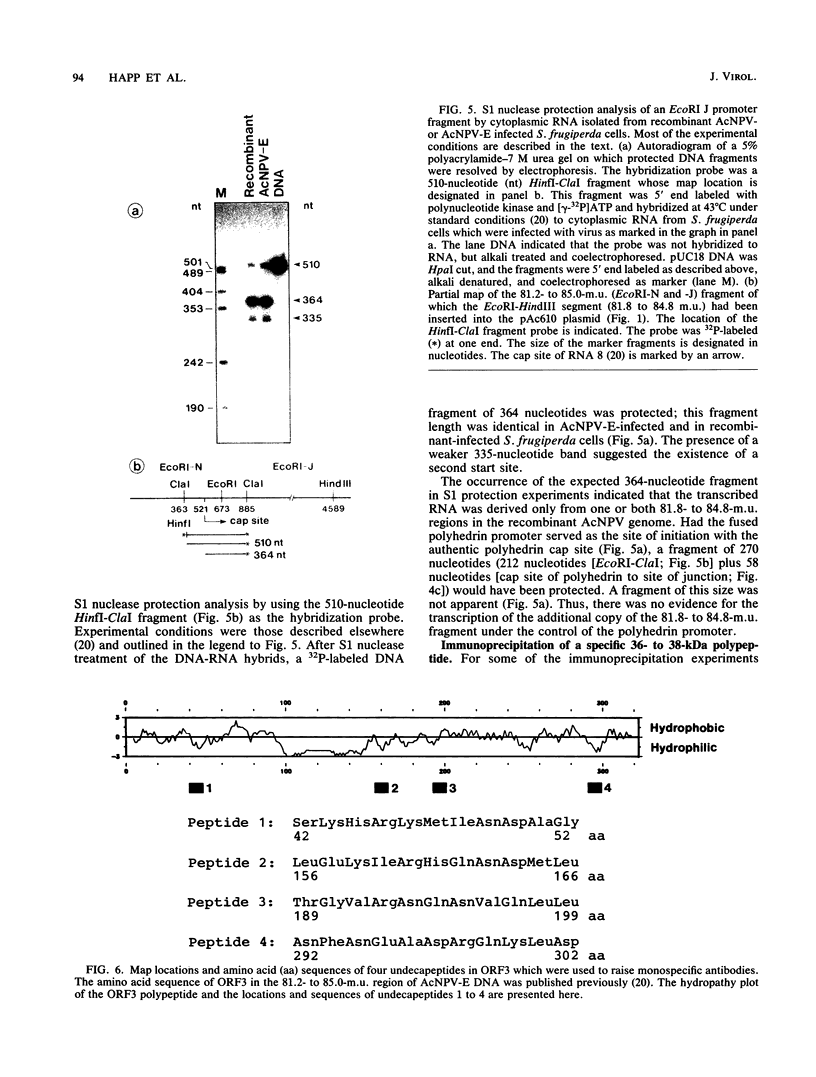
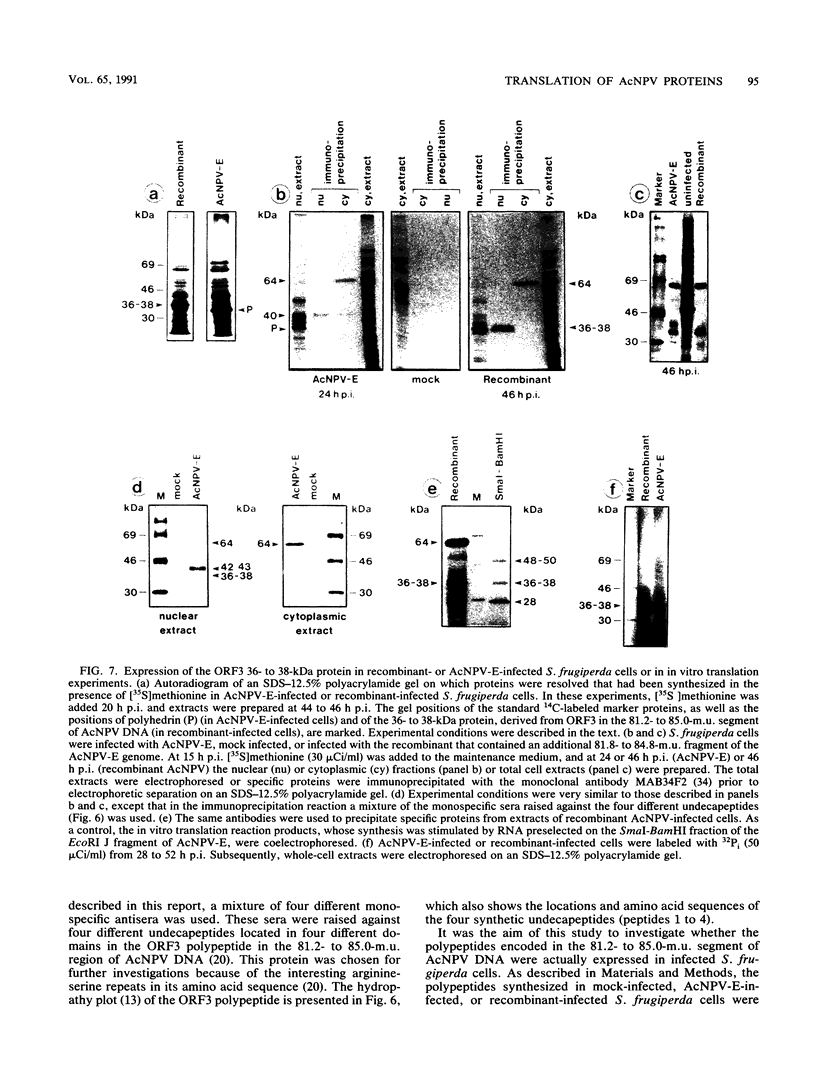
We have previously demonstrated that five open reading frames exist in the nucleotide sequence of the 81.2- to 85.0-map-unit (m.u.) segment of plaque isolate E of Autographa californica nuclear polyhedrosis virus (AcNPV) DNA. The corresponding polypeptides are 9.8, 12.1, 36.6, 25.0, and 48.2 kDa in size (C. Oellig, B. Happ, T. Müller, and W. Doerfler, J. Virol. 63:1494, 1989), and we have investigated whether these proteins can be translated in infected cells. On subfragments of this viral DNA segment, mRNAs were selected from AcNPV-infected Spodoptera frugiperda insect cells at different times postinfection (p.i.). The in vitro translation of these RNAs in a rabbit reticulocyte-derived cell-free translation system yielded polypeptides of approximately 10 to 11, 12 to 14, 28, 36 to 38, and 48 to 50-kDa which were commensurate in size with the theoretically expected values. mRNAs for the 28- and 48- to 50-kDa proteins were identified by their translation products at 6 h p.i., and mRNAs for the 10- to 11-, 12- to 14-, and 36- to 38-kDa proteins were identified by their translation products at 12 h p.i. We constructed an AcNPV recombinant which carried in its polyhedrin gene the 3.9-kbp EcoRI-HindIII (81.8 to 84.8 m.u.) subfragment of the EcoRI J segment. Nucleotide sequence determinations revealed that the intact polyhedrin promoter lay adjacent to the additional 81.8- to 84.8-m.u. fragment in this recombinant. In S. frugiperda cells, which were infected with the recombinant AcNPV, a protein of 36 to 38 kDa was detected at 44 h p.i. in larger amounts than after infection with the nonrecombinant virus. However, there was no evidence for larger amounts of RNA derived from the 81.8- to 84.8-m.u. fragment in recombinant-infected cells. Recombinant-infected cells lacked the polyhedrin polypeptide. The synthesis of the 36- to 38-kDa polypeptide in recombinant- or AcNPV-E-infected S. frugiperda cells could be demonstrated by immunoprecipitation experiments. Peculiarly, this polypeptide was present in the cytoplasm as a 64-kDa glycoprotein. These data corroborate the notion that at least some of the open reading frames encoded in the 81.2- to 85.0-m.u. segment of AcNPV can be expressed in S. frugiperda cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm G. E., Henner D. J. Multiple early transcripts and splicing of the Autographa californica nuclear polyhedrosis virus IE-1 gene. J Virol. 1988 Sep;62(9):3193–3200. doi: 10.1128/jvi.62.9.3193-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esche H., Lübbert H., Siegmann B., Doerfler W. The translational map of the Autographa californica nuclear polyhedrosis virus (AcNPV) genome. EMBO J. 1982;1(12):1629–1633. doi: 10.1002/j.1460-2075.1982.tb01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esche H., Schilling R., Doerfler W. In vitro translation of adenovirus type 12-specific mRNA isolated from infected and transformed cells. J Virol. 1979 Apr;30(1):21–31. doi: 10.1128/jvi.30.1.21-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P. D., Miller L. K. Temporal regulation of baculovirus RNA: overlapping early and late transcripts. J Virol. 1985 May;54(2):392–400. doi: 10.1128/jvi.54.2.392-400.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A. F., Blissard G. W., Rohrmann G. F. Characterization of the genetic organization of the HindIII M region of the multicapsid nuclear polyhedrosis virus of Orgyia pseudotsugata reveals major differences among baculoviruses. J Gen Virol. 1989 Jul;70(Pt 7):1815–1828. doi: 10.1099/0022-1317-70-7-1815. [DOI] [PubMed] [Google Scholar]
- Gombart A. F., Pearson M. N., Rohrmann G. F., Beaudreau G. S. A baculovirus polyhedral envelope-associated protein: genetic location, nucleotide sequence, and immunocytochemical characterization. Virology. 1989 Mar;169(1):182–193. doi: 10.1016/0042-6822(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hauser C., Fusswinkel H., Li J., Oellig C., Kunze R., Müller-Neumann M., Heinlein M., Starlinger P., Doerfler W. Overproduction of the protein encoded by the maize transposable element Ac in insect cells by a baculovirus vector. Mol Gen Genet. 1988 Nov;214(3):373–378. doi: 10.1007/BF00330469. [DOI] [PubMed] [Google Scholar]
- Hooft van Iddekinge B. J., Smith G. E., Summers M. D. Nucleotide sequence of the polyhedrin gene of Autographa californica nuclear polyhedrosis virus. Virology. 1983 Dec;131(2):561–565. doi: 10.1016/0042-6822(83)90522-6. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klenow H., Overgaard-Hansen K., Patkar S. A. Proteolytic cleavage fo native DNA polymerase into two different catalytic fragments. Influence of assay condtions on the change of exonuclease activity and polymerase activity accompanying cleavage. Eur J Biochem. 1971 Oct 14;22(3):371–381. doi: 10.1111/j.1432-1033.1971.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Hauser C., Rott R., Klenk H. D., Doerfler W. Expression of the influenza virus haemagglutinin in insect cells by a baculovirus vector. EMBO J. 1986 Jun;5(6):1359–1365. doi: 10.1002/j.1460-2075.1986.tb04367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lübbert H., Doerfler W. Mapping of Early and Late Transcripts Encoded by the Autographa californica Nuclear Polyhedrosis Virus Genome: Is Viral RNA Spliced? J Virol. 1984 May;50(2):497–506. doi: 10.1128/jvi.50.2.497-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbert H., Doerfler W. Transcription of overlapping sets of RNAs from the genome of Autographa californica nuclear polyhedrosis virus: a novel method for mapping RNAs. J Virol. 1984 Oct;52(1):255–265. doi: 10.1128/jvi.52.1.255-265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbert H., Kruczek I., Tjia S., Doerfler W. The cloned EcoRI fragments of Autographa californica nuclear polyhedrosis virus DNA. Gene. 1981 Dec;16(1-3):343–345. doi: 10.1016/0378-1119(81)90092-5. [DOI] [PubMed] [Google Scholar]
- Mainprize T. H., Lee K., Miller L. K. Variation in the temporal expression of overlapping baculovirus transcripts. Virus Res. 1986 Oct;6(1):85–99. doi: 10.1016/0168-1702(86)90059-6. [DOI] [PubMed] [Google Scholar]
- Oellig C., Happ B., Müller T., Doerfler W. Overlapping sets of viral RNAs reflect the array of polypeptides in the EcoRI J and N fragments (map positions 81.2 to 85.0) of the Autographa californica nuclear polyhedrosis virus genome. J Virol. 1987 Oct;61(10):3048–3057. doi: 10.1128/jvi.61.10.3048-3057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetter C., Oellig C., Doerfler W. An insertion of insect cell DNA in the 81-map-unit segment of Autographa californica nuclear polyhedrosis virus DNA. J Virol. 1990 Apr;64(4):1844–1850. doi: 10.1128/jvi.64.4.1844-1850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. Analysis of baculovirus genomes with restriction endonucleases. Virology. 1978 Sep;89(2):517–527. doi: 10.1016/0042-6822(78)90193-9. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1983 Dec;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tjia S. T., Carstens E. B., Doerfler W. Infection of Spodoptera frugiperda cells with Autographa californica nuclear polyhedrosis virus II. The viral DNA and the kinetics of its replication. Virology. 1979 Dec;99(2):399–409. doi: 10.1016/0042-6822(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., Berg P. Hybridization in situ of SV40 plaques: detection of recombinant SV40 virus carrying specific sequences of nonviral DNA. Science. 1977 Apr 8;196(4286):183–185. doi: 10.1126/science.191907. [DOI] [PubMed] [Google Scholar]
- Vlak J. M., Smith G. E., Summers M. D. Hybridization Selection and In Vitro Translation of Autographa californica Nuclear Polyhedrosis Virus mRNA. J Virol. 1981 Dec;40(3):762–771. doi: 10.1128/jvi.40.3.762-771.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford M., Stewart S., Kuzio J., Faulkner P. Identification and sequence analysis of a gene encoding gp67, an abundant envelope glycoprotein of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol. 1989 Mar;63(3):1393–1399. doi: 10.1128/jvi.63.3.1393-1399.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt M. A., Manning J. S. A phosphorylated 34-kDa protein and a subpopulation of polyhedrin are thiol linked to the carbohydrate layer surrounding a baculovirus occlusion body. Virology. 1988 Mar;163(1):33–42. doi: 10.1016/0042-6822(88)90231-0. [DOI] [PubMed] [Google Scholar]
- Zuidema D., Klinge-Roode E. C., van Lent J. W., Vlak J. M. Construction and analysis of an Autographa californica nuclear polyhedrosis virus mutant lacking the polyhedral envelope. Virology. 1989 Nov;173(1):98–108. doi: 10.1016/0042-6822(89)90225-0. [DOI] [PubMed] [Google Scholar]