Abstract
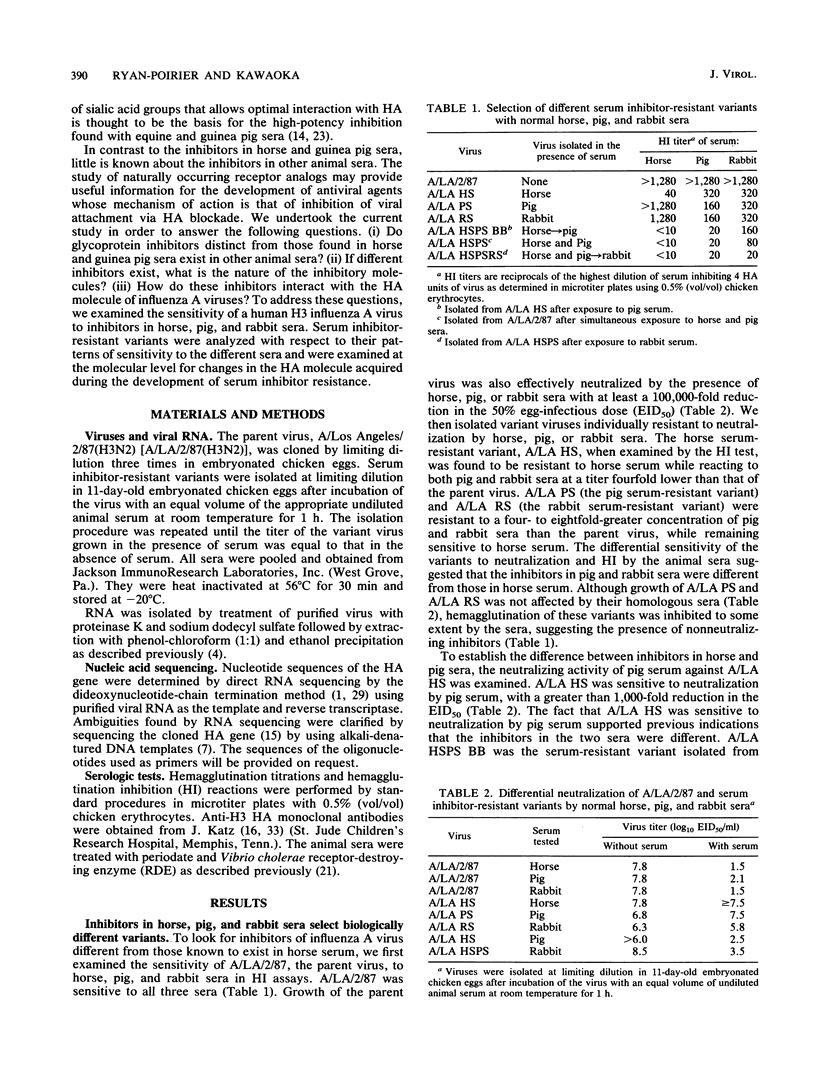
Normal horse and guinea pig sera contain the glycoprotein inhibitor alpha 2-macroglobulin, which inhibits the infectivity and hemagglutinating activity of influenza A viruses of the H2 and H3 subtypes. In the current study, the presence of inhibitors of influenza A virus in pig and rabbit sera was investigated. Variants of influenza virus type A/Los Angeles/2/87(H3N2) that were resistant to horse, pig, or rabbit serum were isolated. Analysis of the variant viruses with anti-hemagglutinin (HA) monoclonal antibodies revealed that antigenic changes occurred with the development of serum inhibitor resistance. Characterization of the inhibitors in pig and rabbit sera by using periodate and receptor-destroying enzyme demonstrated that carbohydrate is an important constituent of the active portion of both inhibitor molecules and that sialic acid is involved in the interaction of the inhibitors with influenza virus HA. Nucleotide sequence analysis of the HA molecule revealed that the serum-resistant variants each acquired a different set of amino acid alterations. The multiply resistant variants maintained the original amino acid changes and acquired additional changes. Sequence modifications in the HA involved the conserved amino acids within the receptor binding site (RBS) at position 137 and the second-shell RBS residues at positions 155 and 186. Amino acid changes also occurred within antigenic site A (position 145) and directly behind the receptor binding pocket (position 220). Amino acid alterations resulted in the acquisition of a potential glycosylation site at position 128 and the loss of potential glycosylation sites at positions 246 and 248. The localization of the amino acid changes in HA1 to the region of the RBS supports the concept of serum inhibitors as receptor analogs. The unique set of mutations acquired by the serum inhibitor-resistant variants strongly suggests that horse, pig, and rabbit sera each contain distinct glycoprotein inhibitors of influenza A virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANANTHANARAYAN R., PANIKER C. K. Non-specific inhibitors of influenza viruses in normal sera. Bull World Health Organ. 1960;22:409–419. [PMC free article] [PubMed] [Google Scholar]
- Air G. M. Nucleotide sequence coding for the "signal peptide" and N terminus of the hemagglutinin from an asian (H2N2) strain of influenza virus. Virology. 1979 Sep;97(2):468–472. doi: 10.1016/0042-6822(79)90358-1. [DOI] [PubMed] [Google Scholar]
- Anders E. M., Hartley C. A., Jackson D. C. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose-binding lectins. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4485–4489. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean W. J., Jr, Sriram G., Webster R. G. Electrophoretic analysis of iodine-labeled influenza virus RNA segments. Anal Biochem. 1980 Feb;102(1):228–232. doi: 10.1016/0003-2697(80)90343-7. [DOI] [PubMed] [Google Scholar]
- COHEN A., BELYAVIN G. Hemagglutination inhibition of Asian influenza viruses: a new pattern of response. Virology. 1959 Jan;7(1):59–74. doi: 10.1016/0042-6822(59)90177-1. [DOI] [PubMed] [Google Scholar]
- Carroll S. M., Higa H. H., Paulson J. C. Different cell-surface receptor determinants of antigenically similar influenza virus hemagglutinins. J Biol Chem. 1981 Aug 25;256(16):8357–8363. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C., Naeve C. W., Webster R. G., Rogers G. N., Paulson J. C. Antigenic analyses of influenza virus haemagglutinins with different receptor-binding specificities. Virology. 1984 Oct 15;138(1):174–177. doi: 10.1016/0042-6822(84)90158-2. [DOI] [PubMed] [Google Scholar]
- Deom C. M., Caton A. J., Schulze I. T. Host cell-mediated selection of a mutant influenza A virus that has lost a complex oligosaccharide from the tip of the hemagglutinin. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3771–3775. doi: 10.1073/pnas.83.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka K., Pritchett T. J., Takasaki S., Kochibe N., Sabesan S., Paulson J. C., Kobata A. 4-O-acetyl-N-acetylneuraminic acid in the N-linked carbohydrate structures of equine and guinea pig alpha 2-macroglobulins, potent inhibitors of influenza virus infection. J Biol Chem. 1989 Jun 15;264(17):9842–9849. [PubMed] [Google Scholar]
- Huddleston J. A., Brownlee G. G. The sequence of the nucleoprotein gene of human influenza A virus, strain A/NT/60/68. Nucleic Acids Res. 1982 Feb 11;10(3):1029–1038. doi: 10.1093/nar/10.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. M., Naeve C. W., Webster R. G. Host cell-mediated variation in H3N2 influenza viruses. Virology. 1987 Feb;156(2):386–395. doi: 10.1016/0042-6822(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Katz J. M., Wang M., Webster R. G. Direct sequencing of the HA gene of influenza (H3N2) virus in original clinical samples reveals sequence identity with mammalian cell-grown virus. J Virol. 1990 Apr;64(4):1808–1811. doi: 10.1128/jvi.64.4.1808-1811.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. M., Webster R. G. Antigenic and structural characterization of multiple subpopulations of H3N2 influenza virus from an individual. Virology. 1988 Aug;165(2):446–456. doi: 10.1016/0042-6822(88)90588-0. [DOI] [PubMed] [Google Scholar]
- Krizanová O., Rathová V. Serum inhibitors of myxoviruses. Curr Top Microbiol Immunol. 1969;47:125–151. doi: 10.1007/978-3-642-46160-6_6. [DOI] [PubMed] [Google Scholar]
- Naeve C. W., Hinshaw V. S., Webster R. G. Mutations in the hemagglutinin receptor-binding site can change the biological properties of an influenza virus. J Virol. 1984 Aug;51(2):567–569. doi: 10.1128/jvi.51.2.567-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett T. J., Brossmer R., Rose U., Paulson J. C. Recognition of monovalent sialosides by influenza virus H3 hemagglutinin. Virology. 1987 Oct;160(2):502–506. doi: 10.1016/0042-6822(87)90026-2. [DOI] [PubMed] [Google Scholar]
- Pritchett T. J., Paulson J. C. Basis for the potent inhibition of influenza virus infection by equine and guinea pig alpha 2-macroglobulin. J Biol Chem. 1989 Jun 15;264(17):9850–9858. [PubMed] [Google Scholar]
- Robertson J. S., Naeve C. W., Webster R. G., Bootman J. S., Newman R., Schild G. C. Alterations in the hemagglutinin associated with adaptation of influenza B virus to growth in eggs. Virology. 1985 May;143(1):166–174. doi: 10.1016/0042-6822(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Daniels R. S., Skehel J. J., Wiley D. C., Wang X. F., Higa H. H., Paulson J. C. Host-mediated selection of influenza virus receptor variants. Sialic acid-alpha 2,6Gal-specific clones of A/duck/Ukraine/1/63 revert to sialic acid-alpha 2,3Gal-specific wild type in ovo. J Biol Chem. 1985 Jun 25;260(12):7362–7367. [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C., Daniels R. S., Skehel J. J., Wilson I. A., Wiley D. C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983 Jul 7;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983 Jun;127(2):361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Pritchett T. J., Lane J. L., Paulson J. C. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology. 1983 Dec;131(2):394–408. doi: 10.1016/0042-6822(83)90507-x. [DOI] [PubMed] [Google Scholar]
- SMITH W., WESTWOOD J. C. N., BELYAVIN G. Influenza; a study of four virus strains isolated in 1951. Lancet. 1951 Dec 29;2(6696):1189–1193. doi: 10.1016/s0140-6736(51)93200-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild G. C., Oxford J. S., de Jong J. C., Webster R. G. Evidence for host-cell selection of influenza virus antigenic variants. Nature. 1983 Jun 23;303(5919):706–709. doi: 10.1038/303706a0. [DOI] [PubMed] [Google Scholar]
- Verhoeyen M., Fang R., Jou W. M., Devos R., Huylebroeck D., Saman E., Fiers W. Antigenic drift between the haemagglutinin of the Hong Kong influenza strains A/Aichi/2/68 and A/Victoria/3/75. Nature. 1980 Aug 21;286(5775):771–776. doi: 10.1038/286771a0. [DOI] [PubMed] [Google Scholar]
- Wang M. L., Katz J. M., Webster R. G. Extensive heterogeneity in the hemagglutinin of egg-grown influenza viruses from different patients. Virology. 1989 Jul;171(1):275–279. doi: 10.1016/0042-6822(89)90538-2. [DOI] [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]


