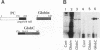Abstract
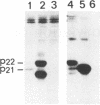
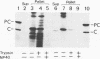
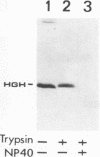
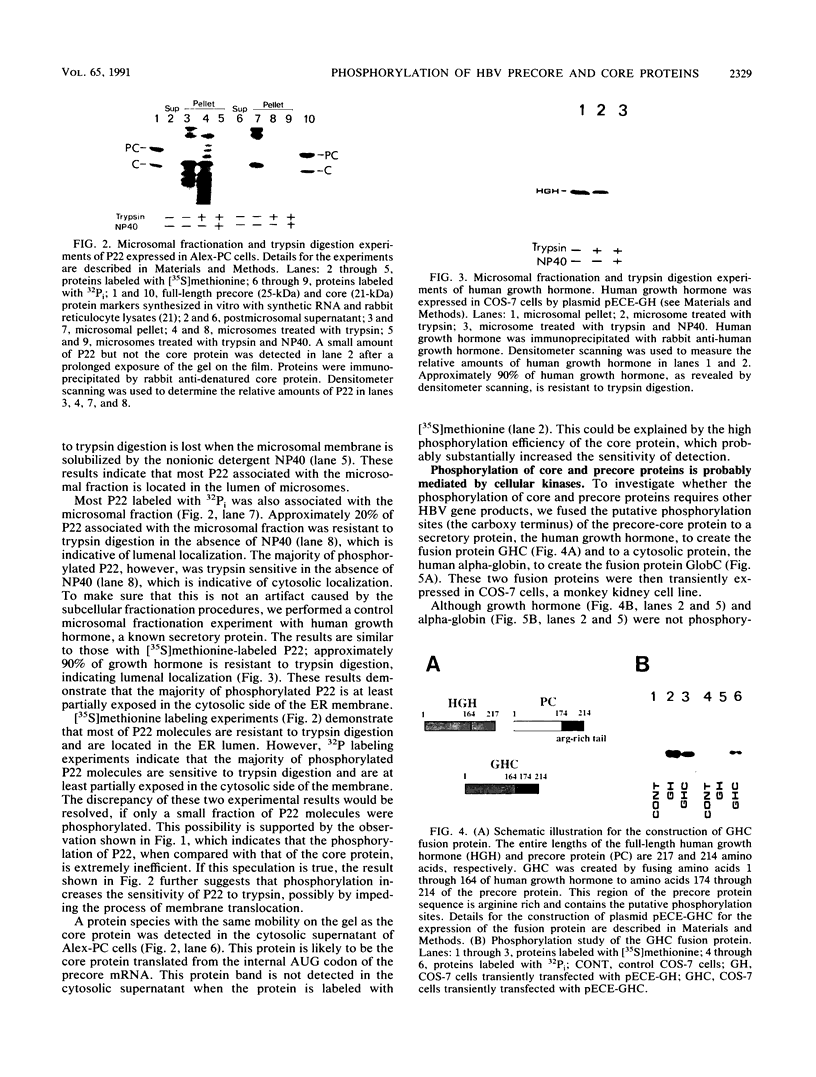
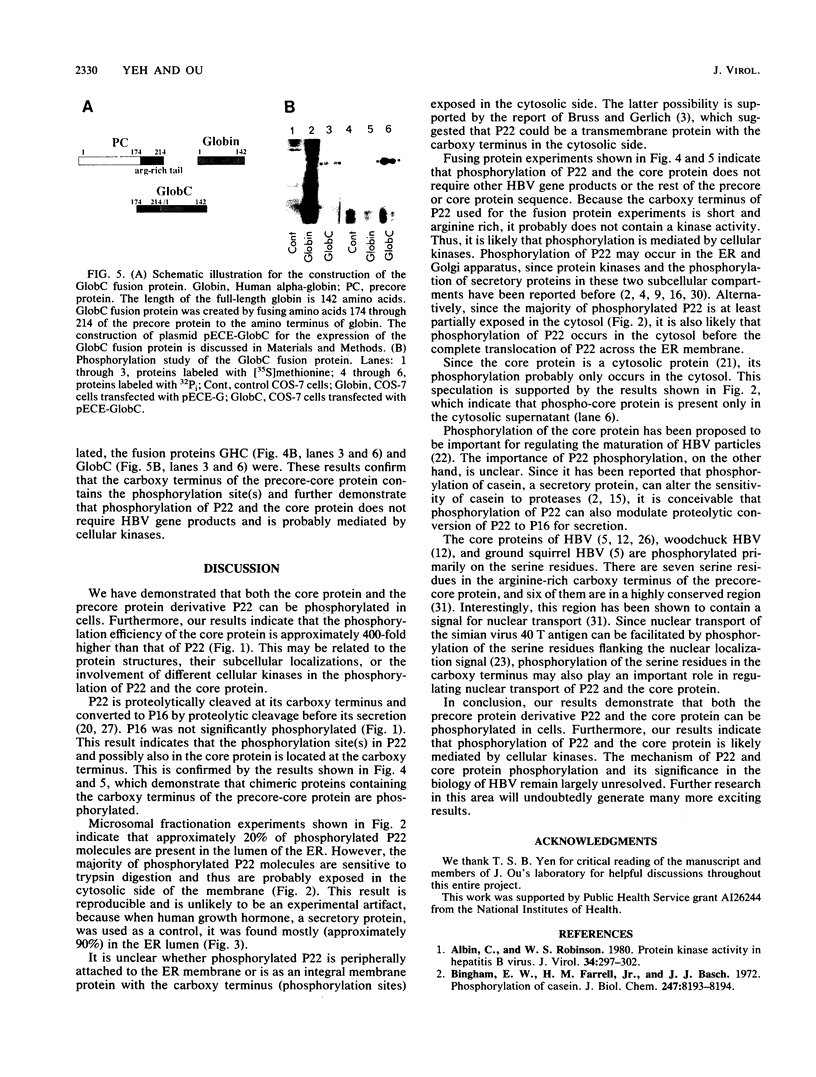
Hepatitis B virus precore and core proteins are related. The precore protein contains the entire sequence of the core protein plus an amino-terminal extension of 29 amino acids. The amino-terminal extension of the precore protein contains a signal sequence for the secretion of the precore protein. This signal sequence is removed after the translocation of the precore protein across the endoplasmic reticulum membrane to produce the precore protein derivative named P22. We demonstrate that both P22 and the core protein can be phosphorylated in cells. Microsomal fractionation and trypsin digestion experiments demonstrate that a fraction of phosphorylated P22 is located in the endoplasmic reticulum lumen. Phosphorylation of P22 likely occurs in the carboxy terminus, since the P22 derivative P16, which lacks the carboxy terminus of P22, is not phosphorylated. Linking the carboxy terminus of the precore-core protein to heterologous secretory and cytosolic proteins led to the phosphorylation of the resulting chimeric proteins. These results indicate that phosphorylation of P22 and the core protein is likely mediated by cellular kinases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin C., Robinson W. S. Protein kinase activity in hepatitis B virus. J Virol. 1980 Apr;34(1):297–302. doi: 10.1128/jvi.34.1.297-302.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham E. W., Farrell H. M., Jr, Basch J. J. Phosphorylation of casein. Role of the golgi apparatus. J Biol Chem. 1972 Dec 25;247(24):8193–8194. [PubMed] [Google Scholar]
- Bruss V., Gerlich W. H. Formation of transmembraneous hepatitis B e-antigen by cotranslational in vitro processing of the viral precore protein. Virology. 1988 Apr;163(2):268–275. doi: 10.1016/0042-6822(88)90266-8. [DOI] [PubMed] [Google Scholar]
- Capasso J. M., Keenan T. W., Abeijon C., Hirschberg C. B. Mechanism of phosphorylation in the lumen of the Golgi apparatus. Translocation of adenosine 5'-triphosphate into Golgi vesicles from rat liver and mammary gland. J Biol Chem. 1989 Mar 25;264(9):5233–5240. [PubMed] [Google Scholar]
- Feitelson M. A., Marion P. L., Robinson W. S. Core particles of hepatitis B virus and ground squirrel hepatitis virus. II. Characterization of the protein kinase reaction associated with ground squirrel hepatitis virus and hepatitis B virus. J Virol. 1982 Aug;43(2):741–748. doi: 10.1128/jvi.43.2.741-748.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Garcia P. D., Ou J. H., Rutter W. J., Walter P. Targeting of the hepatitis B virus precore protein to the endoplasmic reticulum membrane: after signal peptide cleavage translocation can be aborted and the product released into the cytoplasm. J Cell Biol. 1988 Apr;106(4):1093–1104. doi: 10.1083/jcb.106.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich W. H., Goldmann U., Müller R., Stibbe W., Wolff W. Specificity and localization of the hepatitis B virus-associated protein kinase. J Virol. 1982 Jun;42(3):761–766. doi: 10.1128/jvi.42.3.761-766.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glössl J., Hoppe W., Kresse H. Post-translational phosphorylation of proteodermatan sulfate. J Biol Chem. 1986 Feb 5;261(4):1920–1923. [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Junker M., Galle P., Schaller H. Expression and replication of the hepatitis B virus genome under foreign promoter control. Nucleic Acids Res. 1987 Dec 23;15(24):10117–10132. doi: 10.1093/nar/15.24.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R. E., Notvall L. Expression of hepatitis B virus core and precore antigens in insect cells and characterization of a core-associated kinase activity. Virology. 1990 May;176(1):222–233. doi: 10.1016/0042-6822(90)90247-o. [DOI] [PubMed] [Google Scholar]
- Mercier J. C. Phosphorylation of caseins, present evidence for an amino acid triplet code posttranslationally recognized by specific kinases. Biochimie. 1981 Jan;63(1):1–17. doi: 10.1016/s0300-9084(81)80141-1. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Kraft E. L., Jourdian G. W., Van Valen T. R. Phosphorylation of chondroitin sulfate in proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1984 Feb 10;259(3):1720–1726. [PubMed] [Google Scholar]
- Ou J. H., Bell K. D. Comparative studies of hepatitis B virus precore and core particles. Virology. 1990 Jan;174(1):185–191. doi: 10.1016/0042-6822(90)90067-2. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Laub O., Rutter W. J. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1578–1582. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Rutter W. J. Regulation of secretion of the hepatitis B virus major surface antigen by the preS-1 protein. J Virol. 1987 Mar;61(3):782–786. doi: 10.1128/jvi.61.3.782-786.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Yeh C. T., Yen T. S. Transport of hepatitis B virus precore protein into the nucleus after cleavage of its signal peptide. J Virol. 1989 Dec;63(12):5238–5243. doi: 10.1128/jvi.63.12.5238-5243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh J., Zweidler A., Summers J. Characterization of the major duck hepatitis B virus core particle protein. J Virol. 1989 Mar;63(3):1371–1376. doi: 10.1128/jvi.63.3.1371-1376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs H. P., Peters R. Nuclear transport kinetics depend on phosphorylation-site-containing sequences flanking the karyophilic signal of the Simian virus 40 T-antigen. EMBO J. 1989 May;8(5):1479–1484. doi: 10.1002/j.1460-2075.1989.tb03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck M. J., Jameel S., Loukin S. H., Siddiqui A. Expression of hepatitis B viral core region in mammalian cells. Mol Cell Biol. 1986 May;6(5):1393–1400. doi: 10.1128/mcb.6.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck M. J., Siddiqui A. In vivo phosphorylation and protein analysis of hepatitis B virus core antigen. J Virol. 1987 Apr;61(4):955–961. doi: 10.1128/jvi.61.4.955-961.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Schaller H. The secretory core protein of human hepatitis B virus is expressed on the cell surface. J Virol. 1989 Dec;63(12):5399–5404. doi: 10.1128/jvi.63.12.5399-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Masiarz F. R., Rutter W. J. A signal peptide encoded within the precore region of hepatitis B virus directs the secretion of a heterogeneous population of e antigens in Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8405–8409. doi: 10.1073/pnas.85.22.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uy A., Bruss V., Gerlich W. H., Köchel H. G., Thomssen R. Precore sequence of hepatitis B virus inducing e antigen and membrane association of the viral core protein. Virology. 1986 Nov;155(1):89–96. doi: 10.1016/0042-6822(86)90170-4. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Williams D. L. Biosynthesis of the vitellogenins. Identification and characterization of nonphosphorylated precursors to avian vitellogenin I and vitellogenin II. J Biol Chem. 1982 Apr 10;257(7):3837–3846. [PubMed] [Google Scholar]
- Yeh C. T., Liaw Y. F., Ou J. H. The arginine-rich domain of hepatitis B virus precore and core proteins contains a signal for nuclear transport. J Virol. 1990 Dec;64(12):6141–6147. doi: 10.1128/jvi.64.12.6141-6147.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]