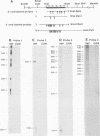
Abstract
Marek's disease is an oncogenic disease of chickens caused by a herpesvirus, Marek's disease virus (MDV). Serial in vitro passage of pathogenic MDV results in amplification of a 132-bp direct repeat in the MDV genome's TRL and IRL repeat regions and loss of tumorigenicity. This led to the hypothesis that upon such expansion, one or more tumor-inducing genes fail to be expressed. In this report a group of cDNAs mapping in the expanded regions were isolated from a pathogenic MDV strain in which the 132-bp direct repeat number was found to range between one and seven. Partial cDNA sequencing and S1 nuclease protection analysis revealed that the corresponding transcripts are either initiated or terminated within or near the expanded regions at multiple sites in both rightward and leftward directions. Furthermore, each 132-bp repeat contains one TATA box and two polyadenylation consensus sequences in each direction. These RNAs contain a partial copy or one or more full copies of the 132-bp direct repeat at either their 5' or 3' end. Northern (RNA) blot analysis showed that the majority of transcripts are 1.8 kb in size, while the minor species range in size from 0.67 to 3.1 kb. Together, these data raise the possibility that the 132-bp direct repeat, and indirectly its copy number, may be involved in the regulation of transcriptional initiation and termination and therefore in the generation of four groups of transcripts from the TRL and IRL, although this remains to be demonstrated.
Full text
PDF



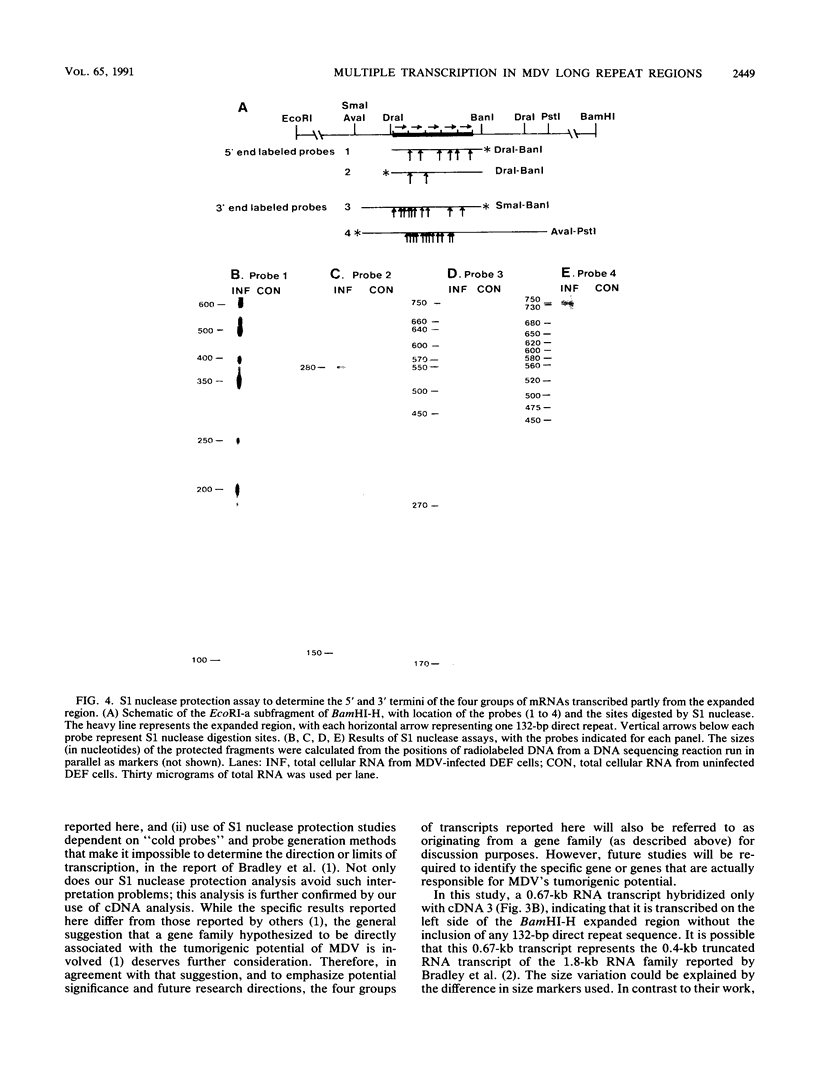
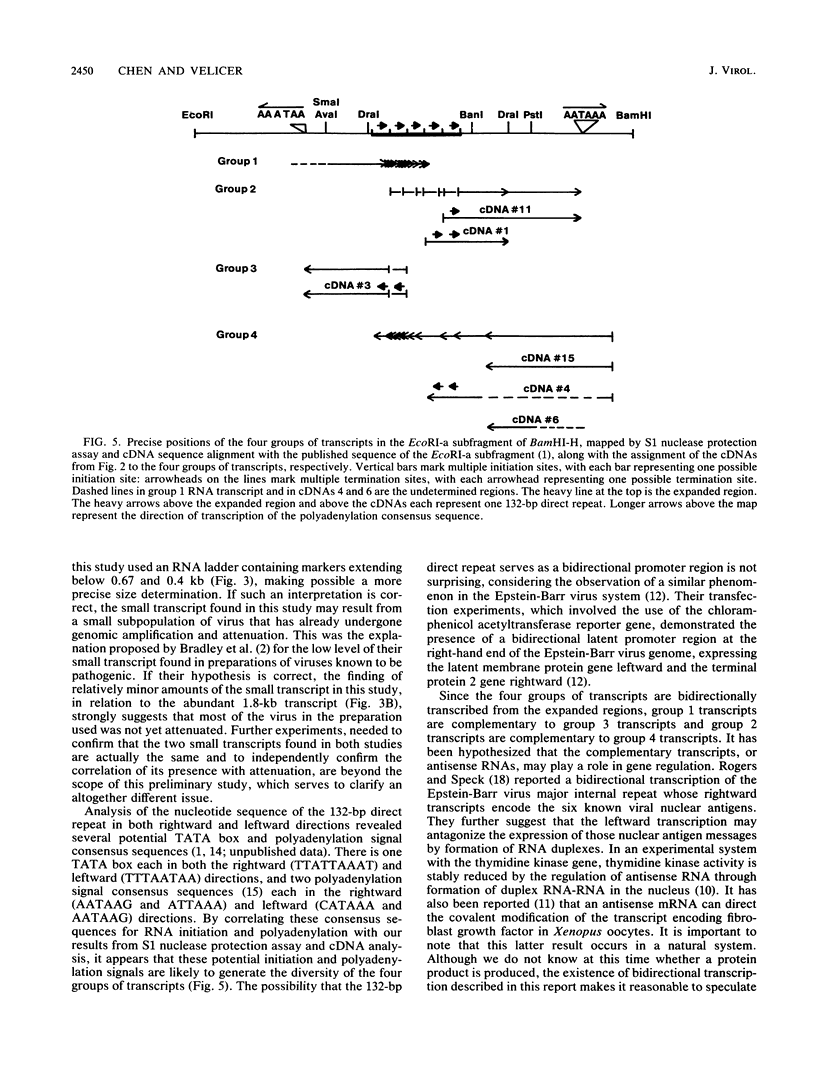

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley G., Hayashi M., Lancz G., Tanaka A., Nonoyama M. Structure of the Marek's disease virus BamHI-H gene family: genes of putative importance for tumor induction. J Virol. 1989 Jun;63(6):2534–2542. doi: 10.1128/jvi.63.6.2534-2542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Lancz G., Tanaka A., Nonoyama M. Loss of Marek's disease virus tumorigenicity is associated with truncation of RNAs transcribed within BamHI-H. J Virol. 1989 Oct;63(10):4129–4135. doi: 10.1128/jvi.63.10.4129-4135.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnek B. W. Marek's disease--a model for herpesvirus oncology. Crit Rev Microbiol. 1986;12(4):293–320. doi: 10.3109/10408418509104432. [DOI] [PubMed] [Google Scholar]
- Churchill A. E., Chubb R. C., Baxendale W. The attenuation, with loss of oncogenicity, of the herpes-type virus of Marek's disease (strain HPRS-16) on passage in cell culture. J Gen Virol. 1969 Jun;4(4):557–564. doi: 10.1099/0022-1317-4-4-557. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Sudo M., Lee Y. S., Tanaka A., Nonoyama M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol. 1984 Jul;51(1):102–109. doi: 10.1128/jvi.51.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Tanaka A., Schierman L. W., Witter R. L., Nonoyama M. The structure of Marek disease virus DNA: the presence of unique expansion in nonpathogenic viral DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(3):751–754. doi: 10.1073/pnas.82.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaubiger C., Nazerian K., Velicer L. F. Marek's disease herpesviruses. IV. Molecular characterization of Marek's disease herpesvirus A antigen. J Virol. 1983 Mar;45(3):1228–1234. doi: 10.1128/jvi.45.3.1228-1234.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R. J., Kung H. J., Velicer L. F. Identification of the gene encoding Marek's disease herpesvirus A antigen. J Virol. 1987 Aug;61(8):2614–2620. doi: 10.1128/jvi.61.8.2614-2620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Hirai K. Marek's disease virus. Adv Virus Res. 1985;30:225–277. doi: 10.1016/s0065-3527(08)60452-2. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. W. An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell. 1989 Nov 17;59(4):687–696. doi: 10.1016/0092-8674(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Laux G., Economou A., Farrell P. J. The terminal protein gene 2 of Epstein-Barr virus is transcribed from a bidirectional latent promoter region. J Gen Virol. 1989 Nov;70(Pt 11):3079–3084. doi: 10.1099/0022-1317-70-11-3079. [DOI] [PubMed] [Google Scholar]
- Maotani K., Kanamori A., Ikuta K., Ueda S., Kato S., Hirai K. Amplification of a tandem direct repeat within inverted repeats of Marek's disease virus DNA during serial in vitro passage. J Virol. 1986 May;58(2):657–660. doi: 10.1128/jvi.58.2.657-660.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Inhibition of RNA cleavage but not polyadenylation by a point mutation in mRNA 3' consensus sequence AAUAAA. Nature. 1983 Oct 13;305(5935):600–605. doi: 10.1038/305600a0. [DOI] [PubMed] [Google Scholar]
- Nazerian K. Attenuation of Marek's disease virus and study of its properties in two different cell cultures. J Natl Cancer Inst. 1970 Jun;44(6):1257–1267. [PubMed] [Google Scholar]
- Rogers R. P., Speck S. H. Bidirectional transcription of the Epstein-Barr virus major internal repeat. J Virol. 1990 May;64(5):2426–2429. doi: 10.1128/jvi.64.5.2426-2429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J. M., Stone H. A. Genetic resistance to Marek's disease. Delineation of the response of genetically resistant chickens to Marek's disease virus infection. Avian Dis. 1972 Jul-Sep;16(4):894–906. [PubMed] [Google Scholar]
- Silva R. F., Witter R. L. Genomic expansion of Marek's disease virus DNA is associated with serial in vitro passage. J Virol. 1985 Jun;54(3):690–696. doi: 10.1128/jvi.54.3.690-696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart P., Ito M., Stewart C., Conrad S. E. Induction of cellular thymidine kinase occurs at the mRNA level. Mol Cell Biol. 1985 Jun;5(6):1490–1497. doi: 10.1128/mcb.5.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]