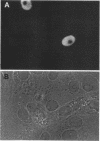
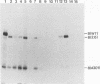
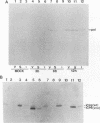
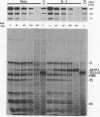
Abstract
We have identified a trans-dominant mutant form of the herpes simplex virus (HSV) DNA-binding protein ICP8 which inhibits viral replication. When expressed by the V2.6 cell line, the mutant gene product inhibited wild-type HSV production by 50- to 150-fold when the multiplicity of infection was less than 5. Production of HSV types 1 and 2 but not production of pseudorabies virus was inhibited in V2.6 cells. The inhibitory effect was not due solely to the high levels of expression, because the levels of expression were comparable to those in the permissive wild-type ICP8-expressing S-2 cell line. Experiments designed to define the block in viral production in V2.6 cells demonstrated (i) that viral alpha and beta gene expression was comparable in the different cell lines, (ii) that viral DNA replication proceeded but was reduced to approximately 20% of the control cell level, and (iii) that late gene expression was similar to that in cells in which viral DNA replication was completely blocked. Genetic experiments indicated that the mutant gene product inhibits normal functions of ICP8. Thus, ICP8 may play distinct roles in replication of viral DNA and in stimulation of late gene expression. The dual roles of ICP8 in these two processes could provide a mechanism for controlling the transition from viral DNA synthesis to late gene expression during the viral growth cycle.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss G. J., Marsden H. S., Hay J. Herpes simplex virus proteins: DNA-binding proteins in infected cells and in the virus structure. Virology. 1975 Nov;68(1):124–134. doi: 10.1016/0042-6822(75)90154-3. [DOI] [PubMed] [Google Scholar]
- Challberg M. D. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. S., Shenk T. The adenovirus DNA-binding protein stimulates the rate of transcription directed by adenovirus and adeno-associated virus promoters. J Virol. 1990 May;64(5):2103–2109. doi: 10.1128/jvi.64.5.2103-2109.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley A. J., Knipe D. M., Jones P. C., Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981 Jan;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., McCarthy A. M., Schaffer P. A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985 Nov;56(2):558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. D., Triezenberg S. J., McKnight S. L. Expression of a truncated viral trans-activator selectively impedes lytic infection by its cognate virus. Nature. 1988 Sep 29;335(6189):452–454. doi: 10.1038/335452a0. [DOI] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Bouchey J., Curtin K., Knipe D. M. Genetic identification of a portion of the herpes simplex virus ICP8 protein required for DNA-binding. Virology. 1988 Apr;163(2):319–329. doi: 10.1016/0042-6822(88)90272-3. [DOI] [PubMed] [Google Scholar]
- Gao M., Knipe D. M. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J Virol. 1989 Dec;63(12):5258–5267. doi: 10.1128/jvi.63.12.5258-5267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss P., Krassa K. B., McPheeters D. S., Nelson M. A., Gold L. Zinc (II) and the single-stranded DNA binding protein of bacteriophage T4. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8515–8519. doi: 10.1073/pnas.84.23.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc Natl Acad Sci U S A. 1986 Jan;83(2):256–260. doi: 10.1073/pnas.83.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A. L., Sandri-Goldin R. M., Levine M., Glorioso J. C. Cloning of herpes simplex virus type 1 sequences representing the whole genome. J Virol. 1981 Apr;38(1):50–58. doi: 10.1128/jvi.38.1.50-58.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haynes L. L., Rothman-Denes L. B. N4 virion RNA polymerase sites of transcription initiation. Cell. 1985 Jun;41(2):597–605. doi: 10.1016/s0092-8674(85)80032-5. [DOI] [PubMed] [Google Scholar]
- Herendeen D. R., Kassavetis G. A., Barry J., Alberts B. M., Geiduschek E. P. Enhancement of bacteriophage T4 late transcription by components of the T4 DNA replication apparatus. Science. 1989 Sep 1;245(4921):952–958. doi: 10.1126/science.2672335. [DOI] [PubMed] [Google Scholar]
- Herendeen D. R., Williams K. P., Kassavetis G. A., Geiduschek E. P. An RNA polymerase-binding protein that is required for communication between an enhancer and a promoter. Science. 1990 May 4;248(4955):573–578. doi: 10.1126/science.2185541. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Holland L. E., Anderson K. P., Shipman C., Jr, Wagner E. K. Viral DNA synthesis is required for the efficient expression of specific herpes simplex virus type 1 mRNA species. Virology. 1980 Feb;101(1):10–24. doi: 10.1016/0042-6822(80)90479-1. [DOI] [PubMed] [Google Scholar]
- Holland L. E., Sandri-Goldin R. M., Goldin A. L., Glorioso J. C., Levine M. Transcriptional and genetic analyses of the herpes simplex virus type 1 genome: coordinates 0.29 to 0.45. J Virol. 1984 Mar;49(3):947–959. doi: 10.1128/jvi.49.3.947-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa F. L., Glorioso J. C., Levine M. A specific 15-bp TATA box promoter element is required for expression of a herpes simplex virus type 1 late gene. Genes Dev. 1988 Jan;2(1):40–53. doi: 10.1101/gad.2.1.40. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., MacLean C., Marsden H. S., Dalziel R. G., Everett R. D. The product of gene US11 of herpes simplex virus type 1 is expressed as a true late gene. J Gen Virol. 1986 May;67(Pt 5):871–883. doi: 10.1099/0022-1317-67-5-871. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Quinlan M. P., Spang A. E. Characterization of two conformational forms of the major DNA-binding protein encoded by herpes simplex virus 1. J Virol. 1982 Nov;44(2):736–741. doi: 10.1128/jvi.44.2.736-741.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Spang A. E. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol. 1982 Jul;43(1):314–324. doi: 10.1128/jvi.43.1.314-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Knipe D. M. An immunoassay for the study of DNA-binding activities of herpes simplex virus protein ICP8. J Virol. 1985 Jun;54(3):731–738. doi: 10.1128/jvi.54.3.731-738.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Knipe D. M. Thermolabile in vivo DNA-binding activity associated with a protein encoded by mutants of herpes simplex virus type 1. J Virol. 1983 Jun;46(3):909–919. doi: 10.1128/jvi.46.3.909-919.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinbach S. S., Casto J. F. Identification and characterization of deoxyribonucleoprotein complexes containing the major DNA-binding protein of herpes simplex virus type 1. Virology. 1983 Dec;131(2):274–286. doi: 10.1016/0042-6822(83)90496-8. [DOI] [PubMed] [Google Scholar]
- Leinbach S. S., Heath L. S. A carboxyl-terminal peptide of the DNA-binding protein ICP8 of herpes simplex virus contains a single-stranded DNA-binding site. Virology. 1988 Sep;166(1):10–16. doi: 10.1016/0042-6822(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Littler E., Purifoy D., Minson A., Powell K. L. Herpes simplex virus non-structural proteins. III. Function of the major DNA-binding protein. J Gen Virol. 1983 May;64(Pt 5):983–995. doi: 10.1099/0022-1317-64-5-983. [DOI] [PubMed] [Google Scholar]
- Orberg P. K., Schaffer P. A. Expression of herpes simplex virus type 1 major DNA-binding protein, ICP8, in transformed cell lines: complementation of deletion mutants and inhibition of wild-type virus. J Virol. 1987 Apr;61(4):1136–1146. doi: 10.1128/jvi.61.4.1136-1146.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. DNA-binding proteins of cells infected by herpes simplex virus type 1 and type 2. Intervirology. 1976;7(4-5):225–239. doi: 10.1159/000149955. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Knipe D. M. Nuclear localization of herpesvirus proteins: potential role for the cellular framework. Mol Cell Biol. 1983 Mar;3(3):315–324. doi: 10.1128/mcb.3.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990 Apr;64(4):1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D. S., Shriver K., Latchman D. S., LaThangue N. B. A filamentous distribution for the herpes simplex virus type 2-encoded major DNA-binding protein. J Gen Virol. 1986 Jul;67(Pt 7):1315–1325. doi: 10.1099/0022-1317-67-7-1315. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Chytil A., Fisher C. M. In vitro characterization of a thermolabile herpes simplex virus DNA-binding protein. J Virol. 1986 Jul;59(1):31–36. doi: 10.1128/jvi.59.1.31-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Weir A. C. Interaction with nucleic acids and stimulation of the viral DNA polymerase by the herpes simplex virus type 1 major DNA-binding protein. J Virol. 1984 Dec;52(3):727–733. doi: 10.1128/jvi.52.3.727-733.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard A. A., Tolentino P., DeLuca N. A. trans-dominant inhibition of herpes simplex virus transcriptional regulatory protein ICP4 by heterodimer formation. J Virol. 1990 Aug;64(8):3916–3926. doi: 10.1128/jvi.64.8.3916-3926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Wang Y. S., Hall J. D. Characterization of a major DNA-binding domain in the herpes simplex virus type 1 DNA-binding protein (ICP8). J Virol. 1990 May;64(5):2082–2089. doi: 10.1128/jvi.64.5.2082-2089.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Lee K. J., Sabourin D. J., Schaffer P. A. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J Virol. 1983 Jan;45(1):354–366. doi: 10.1128/jvi.45.1.354-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. A., Nelson N. J., McGeoch D. J., Challberg M. D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988 Feb;62(2):435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager D. R., Marcy A. I., Coen D. M. Translational regulation of herpes simplex virus DNA polymerase. J Virol. 1990 May;64(5):2217–2225. doi: 10.1128/jvi.64.5.2217-2225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyn Kops A., Knipe D. M. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988 Dec 2;55(5):857–868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]